Microwave Assisted Synthesis and Evaluation of N-Cinnamoyl Aryl Hydrazones for Cytotoxic and Antioxidant Activities
T. Sarala Devi1* and G. Rajitha2
1Department of Pharmaceutical Chemistry, KVSR Siddhartha College of Pharmaceutical Sciences,Vijayawada-520010, Andhra Pradesh, India.
2Institute of Pharmaceutical Technology, Sri Padmavathi Mahila Visvavidyalayam ,Tirupati.
Corresponding Author E-mail: saralaratnakar@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320350
Article Received on : April 08, 2016
Article Accepted on : May 28, 2016
A series of N-cinnamoyl aryl hydrazones 2a-2i were synthesized in good yields by microwave irradiation technique . The title compounds were formed by nucleophilic condensation of various N1- substituted benzylidene-2-cyano aceto hydrazides with N,N-dimethyl amino benzaldehyde. The intermediate N1- substituted benzylidene-2-cyano aceto hydrazide was obtained by condensing various substituted benzaldehydes with cyanoacetohydrazide. The structures of the compounds were characterized by IR, 1H NMR and Mass spectra. The antioxidant activity was studied by DPPH, nitric oxide and hydrogen peroxide methods with ascorbic acid as the standard drug. The compounds were evaluated for cytotoxic activity by BSLT method and their ED50 values were compared with the standard podophyllotoxin. Among the compounds evaluated, N1- benzylidene-2-cyano-3-(4-dimethylamino) phenyl acrylo hydrazide (2a) and N1- (4-methoxy-benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide (2e) showed good antioxidant activity towards all the three models .The compounds 2a and 2e showed ED50 values 3.07 µg/ml and 3.7 µg/ml respectively which were compared against the standard podophyllotoxin (1.64 µg/ml).
KEYWORDS:Cinnamoyl hydrazones; Cyanoacetohydrazide; ED50; BSLT; Cytotoxic; DPPH, Nitric Oxide; Hydrogen Peroxide
Download this article as:| Copy the following to cite this article: Devi T. S, Rajitha G. Microwave Assisted Synthesis and Evaluation of N-Cinnamoyl Aryl Hydrazones for Cytotoxic and Antioxidant Activities. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Devi T. S, Rajitha G. Microwave Assisted Synthesis and Evaluation of N-Cinnamoyl Aryl Hydrazones for Cytotoxic and Antioxidant Activities. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=18160 |
Introduction
Acyl hydrazone derivatives possessing an azometine -NHN=CH- proton are emerging as a novel class of compounds for new lead development 1-4.They were reported to possess, antimicrobial5-6, antitubercular7, antitumour8-9, antioxidant10-12, antiinflammatory13, analgesic14 ,antimalarial 15, anti platelet activities16-17 etc. It was reported that the presence of styryl ketone moiety in the compounds were found to be potent scavengers of free radicals. In view of these observations it was considered of interest to synthesise a new class of acyl hydrazones by incorporating the styryl carbonyl moiety along with aryl hydrazone unit. The aim of present research was to synthesize various N1-(substituted benzylidene)-2-cyano-3-(4-dimethylamino ) phenyl acrylohydrazides and to evaluate for their in-vitro antioxidant and cytotoxic activities.
Materials and Methods
All the chemicals and solvents used in the present study were purchased from Merck, Hi media, S.D. fine Chemicals limited, Mumbai and Sigma Aldrich, USA. Melting points were determined in an open capillary tube in Thermonik precision melting point cum boiling point (C-PMB) apparatus and are uncorrected. Silica gel G coated on laboratory micro slides prepared by dipping method were used. IR spectra (KBr discs) were confirmed by Shimadzu FT-IR spectrophotometer using KBr pellets technique, 1H NMR spectra were recorded on Bruker 300 MHz NMR spectrometer using DMSO as solvent. Mass spectra were recorded on Apex mass spectrophotometer.
Chemistry
General method of synthesis of compounds (1a-1i)
To 0.01 mol of various substituted benzaldehyde , 0.01 mol of cyanoacetohydrazide was added in few ml of ethanol followed by few drops of glacial acetic acid and irradiated in microoven for 1 -3 minutes at 140 watts .The reaction was monitored by TLC and the solid formed was collected and recrystallized from methanol .
General method of synthesis of compounds (2a-2i)
To 0.01 mol of various N1– substituted benzylidene-2-cyanoacetohydrazides ,0.01mol of N,N-di methylamino benzaldehyde was added in few ml of ethanol followed by few drops of pyridine and irradiated in microoven for 1 -3 minutes at 140 watts . The reaction was monitored by TLC and the solid formed is collected and recrystallized from methanol.
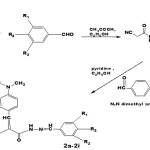 |
Scheme Click here to View Scheme |
The physical data of compounds 2a-2i was tabulated in table-1 .
Table 1: Physical data of N1-benzylidene-2-cyano-3-(4-dimethylamino)phenyl acrylohydrazides (2a-2i)
|
Compound code |
R1 |
R2 RRRRR2 |
R3 |
M.P(OC) |
Yield (%) |
Molecular Formula |
|||||
|
2a |
H |
H |
H |
161-162 |
80 |
C19H18N4 O |
|||||
|
2b |
OCH3 |
OCH3 |
H |
173-176 |
85 |
C21H22N4 O3 |
|||||
|
2c |
OCH3 |
OH |
H |
178-179 |
84 |
C20H18N4 O2 |
|||||
|
2d |
OCH3 |
OCH3 |
OCH3 |
161-166 |
82 |
C22H24N4 O4 |
|||||
|
2e |
H |
OCH3 |
H |
167-175 |
89 |
C20H20N4 O2 |
|||||
|
2f |
H |
OH |
H |
160-162 |
60 |
C19H18N4 O2 |
|||||
|
2g |
H |
4-CH3 |
H |
178-179 |
50 |
C20H20N4 O |
|||||
|
2h |
H |
N(CH3)2 |
H |
158-160 |
49 |
C21H23N4 O |
|||||
|
2i |
3-NO2 |
H |
H |
178-179 |
76 |
C19H17N5 O3 |
|||||
N1-benzylidene-2-cyano-3-(4-dimethylamino) phenyl acrylo hydrazide (2a)
Mol. Formula C19H18ON4, Yield : 80 % ; m.p.: 161-162 0 C ; IR (KBr) cm-1: 3209 (N-H), 3075 (Ar-H), 2258 (C≡N), 1671(C=O), 1551 (C=C) . 1H NMR (300 MHz, DMSO-d6); δ 3.1 (s,6H ,N(CH3)2) , 6.8-7.77 (m,9H, Ar-H) , 8.0(s,IH,C=CH), 8.2(s,IH,N=CH),9.7(s 1H,-CONH); Mass: m/z ( M±1) 318, (M+H)+ 319
N1– (3,4-dimethoxy-benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide (2b)
Mol. Formula C21H22N4O3, Yield : 95 %; m.p.: 173-1760 C ; IR (KBr) cm-1 : 3307 (N-H) , 3068 (Ar-H), 2253 (C≡N) , 1665(C=O), 1566 (C=C). 1H NMR (300 MHz,DMSO- d6) ; δ 3.0 (s,6H ,N(CH3)2) , 3.7-3.8 (s,6H,-OCH3), 6.7-7.7 (m,7H, Ar-H), 7.9(s,IH,C=CH), 8.0 (s,IH,N=CH) , 9.6(s 1H,-CONH)
N1-(3-methoxy,4-hydroxy-benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide( 2c)
Mol. formula C19H18N4O2, Yield : 94%; m.p.:178-1790C ; IR (KBr) cm-1 : 3442 (N-H) , 3078 (Ar-H), 2239 (C≡N), 1653(C=O), 1530 (C=C). 1H NMR (300 MHz,DMSO- d6) ; δ 3.0 (s,6H ,N(CH3)2), 3.7 (s,3H,OCH3), 6.7-6.8 (m,7H,Ar-H) , 7.8 (s,IH,C=CH), 8.0(s,IH,N=CH), 9.6(s ,1H,-CON-H)
N1– (3,4,5-trimethoxy -benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide (2d)
Mol. Formula C22H24N4O4, Yield :92 %; m.p.161-166 0C;IR-(KBr) cm-1 : 3253 (N-H), 3068 (Ar-H), 2319 (C≡N),1660 (C=O) , 1521 (C=C) . 1H NMR (300 MHz,DMSO- d6) ; δ 3.0 (s,6H ,N(CH3) 2) , 3.7-3.8 (s,9H,OCH3), 6.7-8.0(m,6H,Ar-H), 9.6(s, 1H,-CONH), MASS:m/z ( M±1) 408, ( M+H )+ 409
N1– (4-methoxy-benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide (2e)
Mol. formula C20H20N4O2 , Yield :89 %; m.p.167-175 0 C ; IR (KBr) cm-1 : 3213 (N-H) , 3088 (Ar-H), 2320 (C≡N) , 1672 (C=O), 1564 (C=C) .1H NMR (300 MHz,DMSO- d6) ; δ 3.0 (s,6H ,N(CH3)2), 3.7 (s,3H,-OCH3), 6.7-7.6(m, Ar-H), 7.9(s,1H,C=CH) , 8.1(s,1H,N=CH) , 9.6(s, 1H,-CONH)
N1– (4-hydroxy-benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide (2f)
Mol. formula C19H18N4O2 , Yield :60 %; m.p.160-162 0 C ; IR (KBr) cm-1 : 3282 (N-H) , 3095 (Ar-H), 2232 (C≡N) , 1628(C=O), 1553 (C=C) . 1H NMR (300 MHz,DMSO- d6) ; δ 3.0 (s,6H ,N(CH3)2), 6.7-7.5( m,8H,Ar-H), 7.9(s,1H,C=CH) , 8.1(s,1H,N=CH) , 9.6(s ,1H,-CONH)
N1– (4-methyl-benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide (2g)
Mol. formula C20H20N4O, Yield : 50 %; m.p.178-179 0 C ; IR (KBr) cm-1 : 3260 (N-H) , 3100 (Ar-H), 2321 (C≡N) , 1688(C=O), 1511 (C=C) .1H NMR (300 MHz,DMSO- d6) ; δ 1.9 (s,1H,CH 3 ) ,3.0 (s,6H ,N(CH3)2), 6.7-7.7( m,8H,Ar-H), 7.9(s,IH,C=CH) , 8.1(s,IH,N=CH) , 9.6(s ,1H,-CONH)
N1– (4 – dimethylamino -benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide (2h)
Mol. formula C21H23N4O, Yield :89 %; m.p.158-160 0 C ; IR (KBr) cm-1 : 3242(N-H) , 3076 (Ar-H), 2325 (C≡N) , 1683(C=O), 1543 (C=C) .1H NMR (300 MHz,DMSO- d6) ; δ 2.9-3.0 (s,12H ,N(CH3)2), 6.7-7.5(m,8H, Ar-H), 7.9(s,IH,C=CH) , 8.0(s,IH,N=CH) , 9.6(s ,1H,-CONH)
N1– (3-nitro -benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazide (2i)
Mol. formula C19H17N5O3 , Yield :89 %; m.p.178-1790 C ; IR (KBr) cm-1 : 3222 (N-H) , 3086 (Ar-H), 2285 (C≡N) , 1628(C=O), 1513 (C=C) .1H- NMR (300 MHz,DMSO- d6) ; δ 3.0(s,6H ,N(CH3)2), 6.7-7.7( m,8H,Ar-H), 7.9(s,1H,C=CH) , 8.1(s,1H,N=CH) , 9.6(s ,1H,-CONH)
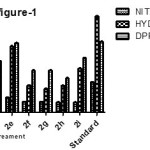 |
Figure Click here to View Figure |
Cytotoxic activity
Brine shrimp lethality test18
Brine Shrimp (Artemia salina) nauplii were hatched in sterile brine solution (prepared using sea salt 38g/L and adjusted the pH to 8.5 using 1N NaOH) under constant aeration for 48 hr. After hatching,10 nauplii were placed in each vial and added various concentrations of drug solutions in a final volume of 5 mL, maintained at 37ºC for 24 h under the light of incandescent lamps and surviving larvae were counted . Each experiment was conducted along with control (vehicle treated), at various concentrations of the test substances. Percentage lethality was determined by comparing the mean surviving larvae of test and control tubes. ED50 values were obtained by using Finney probed analysis software .The result for test compound was compared with the positive control podophyllotoxin.
Antioxidant activity
Determination of nitric oxide Scavenging Activity19
Nitric oxide scavenging activity of samples was determined by the following procedure. 2ml (10mM) of sodium nitro prusside dissolved in 1.5ml phosphate buffer saline (PH-7.4) and 1 ml of different test samples corresponding to 100μM concentration was added in different test tubes respectively and incubated at 25OC for about 150 min. From this 0.5ml was taken and 1ml sulphanilic acid reagent (33% in 20% glacial acetic acid) was added and incubated at room temperature for 5min. 1ml of naphthyl ethylene diamine dihydro chloride (0.1% w/v) was added and again incubated at room temperature for 30min, then measured the absorbance at 540 nm in spectrophotometer.
Determination of the effect of samples on 1, 1-diphenyl-2-picrylhydrazyl (DPPH) Radical 19
DPPH scavenging activity was assessed according to the reported method. Solutions of various test samples at 100µM concentration were added to 100µM DPPH in 95 % ethanol and tubes were kept at an ambient temperature for 20-30 min and absorbance was measured at 517 nm. Ethanol was used as blank and DPPH solution in ethanol served as the control. The effect of Ascorbic acid on DPPH was also assessed for comparison with that of samples.
Determination of Hydrogen Peroxide Scavenging Activity20
4mM solution of H2O2 was prepared in phosphate – buffered saline (PBS, pH 7.4). H2O2 concentration was determined spectrophotometrically from absorbance at 230 nm using molar absorptivity 81 M-1 cm-1 . 1 ml of different samples corresponding to 100μM concentration were added to 0.6ml hydrogen peroxide- PBS solution respectively and control without sample. Absorbance of H2O2 at 230nm was determined 10 minutes later against a blank solution .
Results and Discussion
Chemistry
A series of N1-benzylidene-2-cyano-3-(4-dimethylamino)phenyl acrylohydrazides (2a-2i) were synthesized by two step procedure .In the first step various N1-substituted benzylidene-2-cyanoacetohydrazides were synthesized by taking various substituted aromatic aldehydes and cyanoacetohydrazide in few ml of ethanol by adding a few drops of glacial acetic acid and irradiated in microoven for 1 -3 minutes at 140 watts . The free amino group of cyanoacetohydrazide was condensed with carbonyl group of aldehyde to form schiffs linkage. In the second step the various N1– substituted benzylidene-2-cyanoacetohydrazides are condensed with N,N–dimethylamino benzaldehyde at the electrophilic carbon of cyanoacetohydrazide . The structures of these compounds were established by means of their TLC, IR, 1H NMR and Mass spectra .The synthesized compounds were evaluated for in-vitro antioxidant activity. Among the nine compounds synthesized six were evaluated for in-vitro cytotoxic activity.
Antioxidant activity
The in-vitro antioxidant activity was evaluated by the reported methods of DPPH, nitric oxide and hydrogen peroxide . The results of antioxidant activities of the synthesized compounds were shown in table-2. The unsubstituted (2a ) and 4-methoxy derivatives (2e) showed good scavenging activity towards all the three models at 100µM concentration , when compared with the standard ascorbic acid. From the structure activity relationship studies of the compounds 2a-2i , it was observed that the nature of the substitution on the benzylidene moiety affects the activity. Almost all the compounds showed good to moderate percentage scavenging activity towards DPPH and NO radicals . The moderately electron releasing methoxy derivatives showed good to moderate activity towards H2O2 model. The electron releasing like 4-CH3 and N,N-dimethylamino derivatives showed less scavenging activity towards hydrogen peroxide radical when compared with the standard.
Cytotoxic activity
The brine shrimp lethality test was performed in order to evaluate the cytotoxic nature of the compounds. ED50 values were calculated, based on the percentage of larvae survived at different concentrations of test and standard drugs. Compounds 2a (3.07 µg/ml) and 2e (3.7 µg/ml) showed good ED50.The results are given in table-2. It was observed that the compounds with unsubstituted and 4-methoxy derivatives showed good activity .
Table 2: Antioxidant activity and Cyotoxic activity of compounds (2a-2i)
|
Compound |
% Inhibition of NO |
|
% Inhibition of H2O2 |
% Inhibition of DPPH |
ED50 (µg/ml) |
|
2a |
10 |
59 |
50 |
3.07 |
|
|
2b |
8 |
31 |
36 |
5.28 |
|
|
2c |
11 |
34 |
45 |
6.37 |
|
|
2d |
8 |
39 |
38 |
10.50 |
|
|
2e |
11 |
49 |
51 |
3.77 |
|
|
2f |
8 |
20 |
31 |
– |
|
|
2g |
8 |
18 |
31 |
– |
|
|
2h |
7 |
20 |
25 |
4.07 |
|
|
2i |
7 |
32 |
40 |
– |
|
|
Ascorbic acid |
22 |
71 |
52 |
– |
|
|
Podopyllotoxin |
– |
– |
– |
1.64 |
|
Conclusion
In the present study we have described the synthesis , invitro cytotoxicity screening and antioxidant study of various N1– (substituted benzylidene)-2-cyano-3-(4-dimethylamino) phenyl acrylohydrazides. From the results it was evident that the further substitution and modification on the benzylidene moiety brings a new lead molecule.
Acknowledgements
The authors are thankful to the Siddhartha Academy of General and Technical Education for providing necessary facilities to carry out this research work. The authors are also thankful to laila impex ,vijayawada for providing spectral analysis and laila neutraceuticals for screening of cytotoxic activity.
References
- Sharma, K.R.; Sharma, P.K.; Dixit ,N.S.; Oriental Journal of Chemistry. 2010, 26(1), 69-74.
- Alok, K. Pareek.; Joseph, E.P.; Daya,S.Seth.; Oriental Journal of Chemistry. 2009, 25(1), 159-163.
- Singh, M.; Raghav, N.; International journal of Pharmacy and Pharmaceutical Sciences. 2011, 3(4), 26-32.
- Seleem, S.H.; El-Inany, A.G.; El-Shetary, A.B.; Mousa, A.M.; Chemistry Central Journal. 2011, 5, article 2.
- Abdel-Wahab, F.B.; Awad, A.E.G.; Badria , A.F.; Eur. J. Med. Chem. 2011, 46 (5), 1505-1511.
CrossRef - Ajani, O.O.; Obafemi, C.A.; Nwinyi, O.C.; Akinpelu, D.A.; Bioorg. Med. Chem. 2010, 18(1), 214-221.
CrossRef - Eswaran ,S.; Adhikari, A.V.; Chowdhury, I.H.; Pal,N.K.; Thomas, K.D.; Eur. J. Med. Chem. 2010, 45 (8), 3374-3383.
CrossRef - Cui ,Z.; Li, Y.; Ling, Y.; Huang, J.; Cui, J.; Wang,R.; Yang, X.; Eur. J. Med. Chem. 2010, 45 (12), 5576-5584.
CrossRef - Rafat M. Mohareb; Daisy H. Fleita.; Ola Saka.; molecules-2011, 16, 16-27.
- Musad,E.A.; Mohamed, R.; Saeed, B.Ali.; Vishwanath, B.S.; Rai, K.M.L.; Bioorg. Med. Chem. Lett. 2011, 21(12), 3536–3540.
CrossRef - Rajitha ,G.; Saideepa, N.; Praneetha, P.; Ind. J. Chem B.2011, 50(5), 729-733.
- Gokce Gurkok .; Coban Tulay.; Sibel Suzen.; Journal of Enzyme Inhibition and Medicinal Chemistry, 2009, 24(2), 506–515.
- Radwan, A.A.M.; Ragab ,A.E.; Sabry,M.N.; El-Shenawy, M.S.; Bioorg. Med. Chem. 2007, 15(11), 3832-3841.
CrossRef - Rajitha,G.; Prasad, K.V.S.R.G.; Umamaheswari,A.; Pradhan,D.; Bharathi,K.; Med. Chem. Res., 2014, 23, 5204-5214
CrossRef - Hernandez ,P.; Cabrera, M.; Lavaggi, M.L.; Celano, L.; Tiscornia, I.; da Costa ,T.R.; Thomson, L.; Bollati-Fogolin ,M.;Miranda, A.L.P.; Lima, L.M.; Barreirod, E.J.; Gonzalezd ,M.; Cerecettod, H.; Bioorg. Med. Chem. 2012, 20(6), 2158-2171.
CrossRef - Walcourt, A.; Loyevsky, M.; Lovejoy, D .B.; Gordeuk ,V. R.; Richardson, D. R.; Int J Biochem Cell Biol. 2004, 36, 401.
CrossRef - Giguere, R.J.; Bray, T.L.; Duncan, S.M.; Majetich, G.; Tetrahedron Lett. 1986, 27, 4945.
CrossRef - Krishnaraju, A.V.; Rao T.V.N.; Sundararaju, D.; Vanisree, M.; Tsay, H.S.; Subbaraju, G.V.; International Journal of Applied Science and Engineering ,2006,4(2) ,115-125.
- Sarala Devi,T.; Rajitha,G.; Bharathi, K.; Asian Journal of Chemistry., 2010, 22(7), 5271-5276.
- Vijayabaskaran, M.; Venkateswara Murthy, N.; Babu, G.; Perumal, P.; Jayakar, B.; International Journal of Current Pharmaceutical Research ., 2010, 2(3), ISSN-0975-7066.

This work is licensed under a Creative Commons Attribution 4.0 International License.









