An Aerobic Oxidative Coupling Approach for The Synthesis of N-Substituted 2-Aminobenzothiazole Derivatives using Iron Catalyst.
N. V. Anil Kumar, Shailesha Kamath, Santosh L. Gaonkar and Nitinkumar S. Shetty*
Department of Chemistry, Manipal Institute of Technology, Manipal University, Manipal – 576104, India.
Corresponding Author E-mail: snitinshetty@gmail.com;
DOI : http://dx.doi.org/10.13005/ojc/320349
Article Received on : March 26, 2016
Article Accepted on : April 30, 2016
Article Published : 11 Jun 2016
A facile and convenient method was developed for the formation of novel N substituted 2-aminobenzothiazoles via an iron-catalysed condensation of 2-aminobenzothizole with different amines. This method is applicable for a wide range of aliphatic, aromatic and heterocyclic amines furnishing moderate yields of the corresponding products and thus rendering the methodology as a highly eco-friendly, inexpensive alternative to the existing methods.
KEYWORDS:2-Aminobenzothiazole; N-substituted; synthesis; aerobic oxidative coupling; Iron catalyst
Download this article as:| Copy the following to cite this article: Kumar N. V. A, Kamath S, Gaonkar S. L, Shetty N. S. An Aerobic Oxidative Coupling Approach for The Synthesis of N-Substituted 2-Aminobenzothiazole Derivatives using Iron Catalyst. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Kumar N. V. A, Kamath S, Gaonkar S. L, Shetty N. S. An Aerobic Oxidative Coupling Approach for The Synthesis of N-Substituted 2-Aminobenzothiazole Derivatives using Iron Catalyst. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=17425 |
Introduction
Substituted benzothiazoles exhibits various biological and therapeutical activities.[1, 2]Aminobenzothiazoles are synthesised by employing various catalysts: cobalt was used by Zhu [3, 4], palladium by Vera 5, copper by Saha 6 and Khatun 7, nano copper oxide 8, 9. Other methods of synthesising aminobenzothiazoles is by Herz method10, solid phase synthesis 11, using benzyltrimethylammonium tribromide 12 or sodium dichloroiodate 13, starting from o-nitroaniline 14 or in water 15. Apart from synthesis, following biological activities were reported: Aurora-A kinase inhibitor 16, calcium channel blockers 17, anti-cancer 18, 19, herbicidal activity20, mitochondrial apoptotic inducers 21, neuronal nitric oxide synthase inhibitor22, anti-inflammatory 23, 24, antimalarials 25, antimicrobial 26, 27
Oxidative coupling reactions with amines are reported in literature. Zhou et al 28, 29 used boron-dipyrromethene (BODIPY) under mild condition, Yu et al30 used oxone and trifluoroacetic acid in PEG-400 for the green method. Also, various metals are used for these conversions: gold [31], gold nanoparticle [32], copper [33], silica supported vanadium [34], ruthenium [35] and cobalt[36].
Synthesis of N-substituted amines reported in literature does not use iron as catalyst. In view of these reports and literature, attempt was made to synthesize novel N-substituted aminobenzothiazole derivatives using iron as catalyst via oxidative coupling route.
Results and Discussion
Different iron compounds (FeCl3, FeCl2, FeCl2.2H2O, FeSO4.7H2O, Fe(OAc)2 and FeBr2) were used for the optimisation. The reactions (scheme-1) were carried out with 10% mol of the catalyst and found that FeBr2 results in 63% yield. Therefore, concentration of the catalyst was further reduced to 5% and 2%. This resulted in 64 and 29% yields respectively. Also the effect of the inert medium was evaluated by using N2 and Ar environment and that resulted in 6 and 3% yields respectively. Hence the concentration of FeBr2 was optimised at 5% mol.
 |
Scheme 1: Reaction scheme for the optimisation of the reaction conditions. Click here to View Scheme |
While optimising the concentration of the catalyst, the reactants were used 1.5 equivalents of aminobenzothiazole and 1 equivalent of benzylamine. After finalising the concentration of the catalyst, the reactants concentrations were changed to 1 and 2 equivalents respectively and the yield obtained was only 48%. It has been found that FeBr2 gave better yields amongst all other iron catalysts used. To optimise the conditions, different concentrations were used and 5% mol found to be the optimum concentration without the usage of inert conditions (Table-1). To substantiate our proposed mechanism (scheme – 3), we have carried out the reactions in inert conditions. During the process, oxygen starved environment has yielded very low product.
Table 1: Optimisation Parameters For The Synthesis Of N-Substituted Aminobenzothiazoles.
|
Entry |
Catalyst (% mol) |
Environment, (%mol of 2 amino benzothiazole : %mol of Benzylamine) |
Isolated yield (%) |
|
1 |
FeCl3 (2% mol) |
Air, (1.5:1) |
Traces |
|
2 |
FeCl2 (10% mol) |
Air, (1.5:1) |
Traces |
|
3 |
FeCl2.2H2O (10% mol) |
Air, (1.5:1) |
Not recovered |
|
4 |
FeSO4.7H2O (10% mol) |
Air, (1.5:1) |
Not recovered |
|
5 |
Fe(OAc)2 (10% mol) |
Air, (1.5:1) |
Not recovered |
|
6 |
FeBr2 (10% mol) |
Air, (1.5:1) |
63 |
|
7 |
FeBr2 (5% mol) |
Air, (1.5:1) |
64 |
|
8 |
FeBr2 (2% mol) |
Air, (1.5:1) |
29 |
|
9 |
FeBr2 (10% mol) |
N2, (1.5:1) |
6 |
|
10 |
FeBr2 (10% mol) |
Ar, (1.5:1) |
3 |
|
11 |
FeBr2 (10% mol) |
Air, (2:1) |
48 |
|
12 |
No catalyst |
Air, (1.5:1) |
Not recovered |
Under these optimised conditions, various N-substituted 2-aminobenzothiazoles (3 a – e) were synthesised from their corresponding amine (2 a – e) and 2-amino benzothiazole (1) (scheme – 2). These newly synthesised compounds were characterised by IR, NMR and MS. In order to cross check applicability for different amines, aromatic, aliphatic and heterocyclic substrates were employed and produced moderate yields. Here we are reporting the iron as catalyst via oxidative coupling approach for the synthesis of novel N-substituted aminobenzothiazole derivatives (3 a – e).
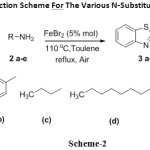 |
Scheme 2: General Reaction Scheme For The Various N-Substituted Aminobenzothiazoles. Click here to View scheme |
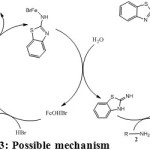 |
Scheme 3: Possible mechanism
|
Experimental Section
General procedure
Iron catalyst was added to a two-necked, round-bottom flask containing the 2-aminobenzothiazole and an amine at room temperature. The resulting reaction mixture was heated at 110o C for 24 h. The progress of the reaction was monitored by TLC. After 24 hours, the reaction mixture was directly purified using column chromatography.
N-benzylbenzo[d]thiazol-2-amine (3a): The title compound was synthesised as per the general method using benzyl amine. Yield: 64%. Mp: 244-246ºC.1H NMR (400 MHz, DMSO-d6): 8.52 (s, 1H, NH), 7.68 – 7.00 (m, 9H, aromatic), 4.60 (s, 2H, CH2). 13C NMR (75 MHz, DMSO -d6): 166.6, 158.8, 152.9, 131.2, 130.8, 129.3, 125.9, 121.4, 118.5, 48.7. Mol Wt: 240.32, m/z: 240.09. Anal. Calcd for C14H12N2S: C, 69.97; H, 5.03; N, 11.66; S, 13.34. Found: C, 70.05; H, 5.29; N, 11.80; S, 12.85.
N-(4-methoxybenzyl)benzo[d]thiazol-2-amine (3b): The title compound was synthesised as per the general method using 4-methoxybenzyl amine.Yield: 67%. Mp: 296-298ºC. 1H NMR (400 MHz, DMSO -d6): 8.43 (s, 1H, NH), 7.67- 6.89 (m, 8H, aromatic), 4.51 (s, 2H, CH2), 3.73 (s, 3H, OCH3).13C NMR (75 MHz, DMSO -d6): 166.6, 158.8, 152.9, 131.2, 130.8, 129.3, 125.9, 121.4, 118.5, 114.2, 55.5, 47.2. Mol Wt: 270.35, m/z: 270.21. Anal. Calcd for C15H14N2OS: C, 66.64; H, 5.22; N, 10.36; S, 11.86. Found: C, 66.91; H, 5.34; N, 10.41; S, 11.46.
N-butylbenzo[d]thiazol-2-amine (3c): The title compound was synthesised as per the general method using n-butyl amine.Yield: 72%. Mp: 199-201ºC. 1H NMR (400 MHz, DMSO – d6): 8.06 (s, 1H, NH), 7.96 – 7.08 (m, 4H, aromatic), 3.56 – 3.48 (t, 2H, NHCH2), 1.52 to 1.33 (m, 4H, CH2-CH2), 0.91 (t, 3H, CH3). 13C NMR (75 MHz, DMSO -d6): 166.6, 153.4, 130.8, 125.8, 121.4, 121.2, 118.4, 47.7, 40.5, 39.7, 39.4. Mol Wt: 206.31, m/z = 206.10. Anal. Calcd for C11H14N2S: C, 64.04; H, 6.84; N, 13.58; S, 15.54. Found C, 64.24; H, 6.99 N, 13.67; S, 15.08.
N-octylbenzo[d]thiazol-2-amine (3d): The title compound was synthesised as per the general method using n-octyl amine.Yield: 75%. Mp: 233-235ºC. 1H NMR (400 MHz, DMSO – d6): 7.99 (s, 1H, NH), 7.65 -6.97 (m, 4H, benzothiozol), 3.36 – 3.31 (t, 2H, NHCH2), 1.61 – 1.26 (m, 12H, CH2), 0.87 – 0.84 (t, 3H, CH3). 13C NMR (75 MHz, DMSO – d6): 166.6, 153.2, 130.7, 125.9, 121.2, 121.2, 118.3, 47.7, 40.8, 40.5, 40.0, 39.7, 39.4, 39.2. Mol Wt: 262.41, m/z = 262.01. Anal. Calcd for C15H22N2S: C, 68.66; H, 8.45; N, 10.68; S, 12.22. Found: C, 68.87; H, 8.57; N, 10.93; S, 11.63.
N-(thiophen-2-ylmethyl)benzo[d]thiazol-2-amine (3e): The title compound was synthesised as per the general method using 2-(Aminomethyl)thiophene.Yield: 59%. Mp: 199-201ºC. 1H NMR (400 MHz, DMSO – d6): 8.22 (s, 1H, NH), 7.51 – 6.98 (m, 7H, aromatic), 4.48 (s, 2H, CH2). 13C NMR (75 MHz, DMSO – d6): 166.6, 158.6, 152.8, 141.2, 131.4, 130.8, 129.3, 126.6, 125.9, 121.4, 118.5, 51.7. Mol Wt: 246.35, m/z = 246.09. Anal. Calcd for C12H10N2S2: C, 58.51; H, 4.09; N, 11.37; S, 26.03. Found: C, 58.67; H, 4.21; N, 11.42; S, 25.69.
Acknowledgement
All authors are grateful for the facilities provided by the Manipal Institute of Technology, Manipal affiliated with Manipal University to carry out this work.
Reference
- Caleta, I., Synthesis, crystal structure and antiproliferative evaluation of some new substituted benzothiazoles and styrylbenzothiazoles. Farmaco, 2004. 59(4): p. 297-305.
CrossRef - Kamal, A., M.A.H. Syed, and S.M. Mohammed, Therapeutic potential of benzothiazoles: a patent review (2010-2014). Expert Opinion on Therapeutic Patents, 2015. 25(3): p. 335-349.
CrossRef - Zhu, T.H., Cobalt-Catalyzed Oxidative Isocyanide Insertion to Amine-Based Bisnucleophiles: Diverse Synthesis of Substituted 2-Aminobenzimidazoles, 2-Aminobenzothiazoles, and 2-Aminobenzoxazoles. Chemistry-a European Journal, 2013. 19(19): p. 5850-5853.
CrossRef - Zhu, T.H., Cobalt(II)-Catalyzed Isocyanide Insertion Reaction with Amines under Ultrasonic Conditions: A Divergent Synthesis of Ureas, Thioureas and Azaheterocycles. Advanced Synthesis & Catalysis, 2014. 356(2-3): p. 509-518.
CrossRef - Vera, M.D. and J.C. Pelletier, Enhanced parallel synthesis efficiency through tandem Pd-catalyzed S- and N-arylation reactions: Single-vessel formation of aminobenzothiazoles. Journal of Combinatorial Chemistry, 2007. 9(4): p. 569-570.
CrossRef - Saha, P., , Ligand-Free Copper-Catalyzed Synthesis of Substituted Benzimidazoles, 2-Aminobenzimidazoles, 2-Aminobenzothiazoles, and Benzoxazoles. Journal of Organic Chemistry, 2009. 74(22): p. 8719-8725.
CrossRef - Khatun, N., , A one-pot strategy for the synthesis of 2-aminobenzothiazole in water by copper catalysis. Rsc Advances, 2012. 2(30): p. 11557-11565.
CrossRef - Gaddam, S.,, Synthesis of N-substituted-2-aminobenzothiazoles using nano copper oxide as a recyclable catalyst under ligand-free conditions, in reusable PEG-400 medium. Chinese Chemical Letters, 2014. 25(5): p. 732-736.
CrossRef - Rout, S.K., An “on-water” exploration of CuO nanoparticle catalysed synthesis of 2-aminobenzothiazoles. Green Chemistry, 2012. 14(9): p. 2491-2498.
CrossRef - Neo, A.G., R.M. Carrillo, and C.F. Marcos, A straightforward synthesis of 2-aminobenzothiazoles from Herz compounds. Organic & Biomolecular Chemistry, 2011. 9(13): p. 4850-4855.
CrossRef - Piscitelli, F., C. Ballatore, and A.B. Smith, Solid phase synthesis of 2-aminobenzothiazoles. Bioorganic & Medicinal Chemistry Letters, 2010. 20(2): p. 644-648.
CrossRef - Jordan, A.D., C. Luo, and A.B. Reitz, Efficient conversion of substituted aryl thioureas to 2-aminobenzothiazoles using benzyltrimethylammonium tribromide. Journal of Organic Chemistry, 2003. 68(22): p. 8693-8696.
CrossRef - Telvekar, V.N., H.M. Bachhav, and V.K. Bairwa, A Novel System for the Synthesis of 2-Aminobenzthiazoles using Sodium Dichloroiodate. Synlett, 2012(15): p. 2219-2222.
CrossRef - Mirza, B., R. Mirzazadeh, and M. Zeeb, A new and efficient synthesis of 2-aminobenzothiazoles derivatives from o-nitroaniline. Journal of Chemical Research, 2013(12): p. 778-779.
CrossRef - Zhang, X.Y., An economically and environmentally sustainable synthesis of 2-aminobenzothiazoles and 2-aminobenzoxazoles promoted by water. Green Chemistry, 2011. 13(2): p. 413-418.
CrossRef - Katari, N.K., M. Venkatanarayana, and K. Srinivas, Dithiocarbamate promoted practical synthesis of N-Aryl-2-aminobenzazoles: Synthesis of novel Aurora-A kinase inhibitor. Journal of Chemical Sciences, 2015. 127(3): p. 447-453.
CrossRef - Kalavagunta, P.K., et al., Design and green synthesis of 2-(diarylalkyl)aminobenzothiazole derivatives and their dual activities as angiotensin converting enzyme inhibitors and calcium channel blockers. European Journal of Medicinal Chemistry, 2014. 83: p. 344-354.
CrossRef - Gardner, C.R.,, Synthesis of retinoid enhancers based on 2-aminobenzothiazoles for anti-cancer therapy. Bioorganic & Medicinal Chemistry, 2012. 20(23): p. 6877-6884.
CrossRef - Kamal, A., , Synthesis and Biological Evaluation of Mercapto Triazolo-Benzothiadiazine Linked Aminobenzothiazoles as Potential Anticancer Agents. Chemical Biology & Drug Design, 2009. 73(6): p. 687-693.
CrossRef - Fajkusova, D., Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles. Bioorganic & Medicinal Chemistry, 2012. 20(24): p. 7059-7068.
CrossRef - Kamal, A., 2-Anilinonicotinyl linked 2-aminobenzothiazoles and [1,2,4]triazolo[1,5-b] [1,2,4]benzothiadiazine conjugates as potential mitochondrial apoptotic inducers. Bioorganic & Medicinal Chemistry, 2011. 19(23): p. 7136-7150.
CrossRef - Patman, J., , Novel 2-aminobenzothiazoles as selective neuronal nitric oxide synthase inhibitors. Bioorganic & Medicinal Chemistry Letters, 2007. 17(9): p. 2540-2544.
CrossRef - Khedekar, P.B., Synthesis and anti-inflammatory activity of alkyl/arylidene-2-aminobenzothiazoles and 1-benzothiazol-2-yl-3-chloro-4-substituted-azetidin-2-ones. Arzneimittel-Forschung-Drug Research, 2003. 53(9): p. 640-647.
- Velingkar, V.S., Synthesis of Substituted 2-Aminobenzothiazoles as Non-Acidic Antiinflammatory Agents. Indian Journal of Heterocyclic Chemistry, 2010. 19(4): p. 415-416.
- Atkinson, E.R. and F.E. Granchelli, Antimalarials V: aminobenzothiazoles. J Pharm Sci, 1976. 65(4): p. 618-20.
CrossRef - El-Shaaer, H.M., , Synthesis, antimicrobial activity and bleaching effect of some reaction products of 4-oxo-4H-benzopyran-3-carboxaldehydes with aminobenzothiazoles and hydrazides. Farmaco, 1998. 53(3): p. 224-232.
CrossRef - Annadurai, S., Design and synthesis of 2-aminothiazole based antimicrobials targeting MRSA. Bioorganic & Medicinal Chemistry Letters, 2012. 22(24): p. 7719-7725.
CrossRef - Zhou, Y., et al., Synthesis and properties of BODIPY polymers and their photocatalytic performance for aerobic oxidation of benzylamine. Catalysis Communications, 2015. 64: p. 96-100.
CrossRef - Zhou, Z. and W. Yang, Syntheses of 2-Aryl Benzothiazoles via Photocatalyzed Oxidative Condensation of Amines with 2-Aminothiophenol in the presence of Bodipy derivatives. Synthetic Communications, 2014. 44(21): p. 3189-3198.
CrossRef - Yu, F.-C.,, An atom-economic green approach: oxidative synthesis of functionalized 1,4-dihydropyridines from N,N-dimethylenaminones and amines. Tetrahedron Letters, 2015. 56(6): p. 837-841.
CrossRef - Opris, C.M., New multicomponent catalysts for the selective aerobic oxidative condensation of benzylamine to N-benzylidenebenzylamine. Catalysis Science & Technology, 2014. 4(12): p. 4340-4355.
CrossRef - Grirrane, A., A. Corma, and H. Garcia, Highly active and selective gold catalysts for the aerobic oxidative condensation of benzylamines to imines and one-pot, two-step synthesis of secondary benzylamines. Journal of Catalysis, 2009. 264(2): p. 138-144.
CrossRef - Xiao, T.,, Copper-catalyzed synthesis of benzazoles via aerobic oxidative condensation of o-amino/mercaptan/hydroxyanilines with benzylamines. Rsc Advances, 2013. 3(36): p. 15592-15595.
CrossRef - Rao, K.T.V., Vapor-phase selective aerobic oxidation of benzylamine to dibenzylimine over silica-supported vanadium-substituted tungstophosphoric acid catalyst. Green Chemistry, 2013. 15(3): p. 837-846.
CrossRef - Fan, X., Ru(III)-catalyzed oxidative reaction in ionic liquid: an efficient and practical route to 2-substituted benzothiazoles and their hybrids with pyrimidine nucleoside. Tetrahedron Letters, 2010. 51(27): p. 3493-3496.
CrossRef - Zhou, X.T., Oxidative condensation reactions of (diethylenetriamine)cobalt(III) complexes with substituted bis(pyridin-2-yl)methane ligands. Journal of Molecular Structure, 2005. 740(1-3): p. 91-100.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









