A Sensitive and Selective GC-MS Method for Analysis of Genotoxic Impurities in Dobutamine Hydrochloride
Vijaya Lakshmi Maddala*1,2, P. C. Ray1 and K. M. V. N Rao1
1Inogent Laboratories Private Limited.
2Jawaharlal Nehru Technological University, Hyderabad.
Corresponding Author E -mail: Vijaya_Chem@Yahoo.Com
DOI : http://dx.doi.org/10.13005/ojc/320347
Article Received on : April 06, 2016
Article Accepted on : May 27, 2016
A sensitive GC-MS method for analysis of residues of Methyl-p-toluene sulfonate [Fig.2], Ethyl p-toluene sulfonate [Fig.3] and Isopropyl p-toluene sulfonate [Fig.4] Genotoxic impurities in Dobutamine hydrochloride [Fig.1] drug substance, has been developed and validated. Under the optimum conditions of the proposed method is specific, precise, linear, accurate and rugged. The response was linear in the concentration range of LOQ to 0.18 mg.g-1 with correlation coefficients of greater than 0.99 for each impurity. Under the optimum conditions used for the sample preparation the recovery of each impurity is in between 78-108%
KEYWORDS:Dobutamine hydrochloride; GC-MS; Genotoxic impurities; method validation
Download this article as:| Copy the following to cite this article: Maddala V. L, Ray P. C, Rao K. M. V. N. A Sensitive and Selective GC-MS Method for Analysis of Genotoxic Impurities in Dobutamine Hydrochloride. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Maddala V. L, Ray P. C, Rao K. M. V. N. A Sensitive and Selective GC-MS Method for Analysis of Genotoxic Impurities in Dobutamine Hydrochloride. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=17838 |
Introduction
Genotoxic impurities have the tendency to cause a potential damage of the genetic material, for example DNA even at very low level of exposure. There are three primary effects that genotoxins can have on organisms by effecting their genetic information. Genotoxins can be carcinogens, or cancer causing agents, mutagens or mutations- causing agents or teratogens, birth defect causing agents. Thus, there is a high demand and need to identify the potential impurities that arise during synthesis, purification and storage keeping the damaging effects of Genotoxic impurities, many international regulatory agencies has already published the guidelines regarding the limits of Genotoxic impurities. Both EMEA guidelines and a phRMA propose a maximum of daily exposure target of 1.5µg per day genotoxic impurities in pharmaceuticals is recommended Based on the Threshold of toxicological concern (TTC), the concentration limits of the impurity in a drug substances or drug products can be derived based on the maximum daily dose: concentration (ppm) = [1.5µg/ day]/[dose (g/day)]. For a drug dosed at 1g per day, for example, 1.5 ppm would be the limit of that specific Genotoxic impurity.
Dobutamine hydrochloride(1-2) is a sympathomimetic with direct effects on beta1- adrenergic receptors, giving it a prominent isotropic action on the heart. It also has some alpha – and beta2 – agonist properties. Although it is structurally related to dopamine it has no specific dopaminergic properties; however, like dopamine, the isotropic action of dobutamine on the heart is associated with less cardiac – accelerating effect than that of isoprenaline. Dobutamine is used to increase the contractility of the heart in acute heart failure, as occurs in cardiogenic shock and myocardial infarction. It is also used in septic shock. Other circumstances in which its isotropic activity may be useful are during cardiac surgery and positive end – expiratory pressure ventilation.
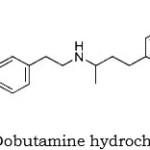 |
Figure 1: Dobutamine hydrochloride |
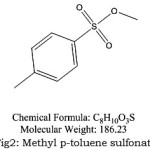 |
Figure 2: Methyl p-toluene sulfonate |
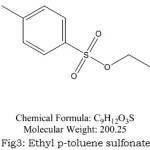 |
Figure 3: Ethyl p-toluene sulfonate Click here to View Figure |
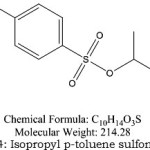 |
Figure 4: Isopropyl p-toluene sulfonate |
During the synthesis these are the three Genotoxic impurities present in the Dobutamine hydrochloride. Based on the literature survey, to the best of our knowledge there is no method to estimate of these Genotoxic impurities. Here in this paper we present a sensitive GC-MS3 method has been developed and validated for the estimation of Genotoxic impurities. The mentioned Genotoxic impurities are prepared and tested in Inogent Laboratories private limited, Nacharam, Hyderabad.
Experimental
Active pharmaceutical ingredient standards and samples were supplied by Inogent Laboratories private Limited, Hyderabad, India. The HPLC grade Toluene was purchased from Merck, Darmstadt, Germany.
Chromatographic conditions and equipment
Mutagenic impurities5-6 are separated by GCMS on HP-5 ms 30mX0.25mm, 0.25µ column, Samples and standards are prepared in 0.1% Diethyl amine in methanol in the system Agilent 7890B Gas chromatograph equipped with 5977A Mass selective detector. The oven programme consists of Initial the oven is programmed at 60°C for 3 minutes, then increased at the rate of 25°C/min upto 250°C, hold the temperature at 250°C for 10.5 minutes, Injector temperatue is put at 200°C, Auxiliary temperature at 300°C. The carrier gas is Helium(High purity 99.999%), at a constant flow rate of 1.4 mL/min and the split ratio is 1:10. having the injection volume 1.0 µL, the total runtime of 21 minutes.
The Ion source in the mass spectrophotometer is the Electron impact ionization, in which the acquisition mode is SIM(Selective ion monitoring), having the source temperature at 230°C and quadrapole temperature at 150°C, solvent delay time is put for 7 minutes. The SIM parameters are Group id is 1, high resolution and the ions/dwell time are 155/100, 172/100, 186/100, 200/100, 214/100.
Preparation of the stock solutions
An individual stock solution (1000 µg.mL-1) of all impurities was prepared in diluent. Sample solution was prepared (10 mg.mL-1) in diluent. Limit of detection and limit of quantification for the impurities were evaluated by preparing the impurities in the level from 60 µg mL-1 to 150 µg mL-1. Linearity of each impurity is determined from LOQ level to 180 µg mL-1.
Results and Discussion
Method development
Method development was planned for the estimation of the above mentioned Genotoxic impurities in Dobutamine hydrochloride by using Gas chromatographic technique with mass spectrophotometer as detector, as evaluation limit was as low as 120 µg mL-1. Different columns with different stationary phases and dimensions were used like DB-624, DB-1, RTX-624 and RTX-1301 for the analysis. Finally separation is achieved on HP-5 MS column, mid polar column with 5% phenyl and 95% dimethyl polysiloxane as a stationary phase. During the method development using this column, appropriate separation is achieved between the impurities.
 |
Figure 5 Click here to View Figure |
Method validation
Method validation11 activity was conducted with necessary parameters as per the analytical method validation guidelines of International conference on Harmonization.
Specificity
Specificity is done satisfactorily by checking the all impurities individually and with spiked solution, confirmed that there is no interference of any impurity.
Limit of detection (LOD) and Limit of Quantification (LOQ)
The LOD and LOQ values for each impurity were predicted by using the series of solutions from 60 µg mL-1 to 150 µg mL-1 by using standard deviation of the response and slope method. The limit of detection and limit of quantification results are as shown below table-1. The method is precise at Limit of quantification level. The respective results are represented in below table-2
Table 1: LOD/LOQ
|
LOD/LOQ |
Methyl p- toluene sulfonic acid |
Ethyl p- toluene sulfonic acid |
Isopropyl p- toluene sulfonic acid |
|
Limit of quantification (ppm) |
13 |
8 |
12 |
|
Limit of detection(ppm) |
41 |
24 |
36 |
Table 2: Precision at limit of quantification level
|
Parameter |
Methyl p- toluene sulfonic acid |
Ethyl p- toluene sulfonic acid |
Isopropyl p- toluene sulfonic acid |
|
%RSD for six preparations |
3.3 |
5.9 |
5.7 |
Linearity9
Linearity of the three impurities were satisfactorily done. A series of solutions were prepared at test concentration levels from around LOQ level to180 µg mL-1 level. The peak area versus concentration data was done by linearity plot slope, intercept and residual sum of squares analysis. The calibration curve given based on response over the concentration range of the impurities. The results are tabulated in table-3
Table 3: Linearity
|
Parameter |
Methyl p- toluene sulfonic acid |
Ethyl p- toluene sulfonic acid |
Isopropyl p- toluene sulfonic acid |
|
Correlation coefficient |
0.997 |
0.997 |
0.997 |
|
Slope |
262.993 |
612.167 |
558.855 |
|
Y-Intercept |
2746.472 |
-525.528 |
-6081.424 |
Precision
The precision8 of the developed method was checked by preparing the solutions by spiking the impurities at 100% level(120 µg mL-1) with the drug substance for six times to show that the system is precise. The %Relative standard deviation of the areas at each level is less than 8% confirming the good precision. The method is also proved as rugged by analyzing the same with different column and different analyst on different day. The results are represented in table-5
Table 4: Accuracy
|
Accuracy Level |
%Recovery |
||
|
Methyl p- toluene sulfonic acid |
Ethyl p- toluene sulfonic acid |
Isopropyl p- toluene sulfonic acid |
|
|
LOQ Level |
96.9 |
84.7 |
89.1 |
|
50% level(60 ppm) |
96.0 |
94.4 |
90.7 |
|
100% level(120 ppm) |
83.9 |
91.4 |
89.8 |
|
150% level(180 ppm) |
87.2 |
78.3 |
92.3 |
Table 5: Method precision and Ruggedness
|
Parameter |
Methyl p- toluene sulfonic acid |
Ethyl p- toluene sulfonic acid |
Isopropyl p- toluene sulfonic acid |
|
Method precision %RSD for six preparations |
4.5 |
2.7 |
3.2 |
|
Ruggedness % RSD for six preparations |
5.7 |
6.9 |
7.1 |
Accuracy
The accuracy of the method was evaluated in sample solutions were prepared in triplicate by spiking all impurities at LOQ level, 60 µg mL-1 , 120 µg mL-1 and 150 µg mL-1. The % recoveries of the impurities are in table-4
Conclusion
In this paper a sensitive specific, accurate, validated and well defined GC-MS method for the quantification of Genotoxic impurities in Dobutamine hydrochloride at ppm level was described. The described method is highly reliable technique for the quantification of impurities present in the Dobutamine hydrochloride.
References
- Thippani ramesh, Nageswararao pothuraju, Ramisetty Nageswararao, saida shaik, Journal of pharmaceutical analysis, 2013, 3 434-439.
- Kondamadgu S, Gandu V. Quantification of Drugs and Pharmaceuticals Using N-Bromosuccinimide and Methyl. Orient J Chem 2016;32(1).
- Sahil K, Prashant B, Akanksha M, Premjeet S, Devashish R ()GC-MS: Applications. International Journal Pharma & Biological Archives 2011 2: 1544-1560.
- Senthamil Selvan P, Veeran Gowda K, Mandal U, SamSolomon WD, Pal TK, J Chromatogr B, 2007, 858, .,143-150.
- Kamila MM, Mondal N, Ghosh LK, Gupta BK, Pharmazie, 2007, 62,486-487.
- Maurer HH, Tenberken O, Kratzch C, Weber AA, Peters FT J Chromatogr A, 2004 , 169-181.
CrossRef - Ramakrishna NVS, Vishwottam KN, Koteshwara MS,Santosh MM, Varma DP, J Pharm Biomed Anal, , 2005, 39, 1006-1013.
CrossRef - http://www.emea.eu.int/pdfs/human/swp/519902en.pdf .
- http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079235.pdf.
- M. Sun, L. Bai, and D. Q. Liu, Journal of Pharmaceutical & Biomedical Analysis, 2009.49(2): p.529-533.
CrossRef - International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (I.C.H.), Q2/R1, Validation of analytical procedures Text and methodology, 1995.

This work is licensed under a Creative Commons Attribution 4.0 International License.









