Synthesis and Corrosion Inhibition Study of Benzothiazepine Derivatives on Mild Steel In Acid Medium
T. Sasikala1,K. Parameswari2 and S.Chitra1
1Department of Biotechnology, Hindusthan College of Arts and Science, Coimbatore-28. India 2Department of Chemistry, P.S.G.R. Krishnammal College for Women, Coimbatore-04.India Corresponding author email: sasikanagu@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/320248
Article Received on :
Article Accepted on :
Article Published : 12 Apr 2016
2-ethoxy-4-(4-phenyl-2, 3-dihydro-1, 5-benzothiazepin-2-yl) phenol (EPBTZ) and 2-(4-methoxyphenyl)-4-phenyl-2, 3-dihydro-1, 5-benzothiazepine (MPPBTZ) were synthesized by the condensation reaction between o-aminothiophenol and chalcone. The synthesized benzothiazepines were characterized by FTIR spectra. Their corrosion inhibition property on mild steel in sulphuric acid medium was investigated by weight loss and electrochemical techniques. Scanning electron microscopic studies were employed to examine the surface morphology of the inhibited and uninhibited metal samples. The compound EPBTZ revealed good corrosion protection property than MPPBTZ at all the temperatures studied. Electrochemical studies showed that the inhibitors behave as mixed type inhibitor retarding both cathodic and anodic corrosion reaction by forming an adsorbed protective layer.
KEYWORDS:Mild steel; benzothiazepine; corrosion inhibitors; impedance; polarization
Download this article as:| Copy the following to cite this article: Sasikala T, Parameswari K, Chitra S. Synthesis and Corrosion Inhibition Study of Benzothiazepine Derivatives on Mild Steel in Acid Medium. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Sasikala T, Parameswari K, Chitra S. Synthesis and Corrosion Inhibition Study of Benzothiazepine Derivatives on Mild Steel in Acid Medium. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15274 |
Introduction
Corrosion of metals poses serious economic challenge to industries. Mild steel finds prominent application as structural material which is prone to corrosion in acidic medium. This problem paid the interest to corrosion scientists and engineers globally, to understand the mechanism and phenomenon of corrosion and to find appropriate solution to inhibit corrosion. Use of organic compounds containing heteroatoms and aromatic rings is one of the practical methods to inhibit corrosion 1.
Benzothiazepine received the attention of many researchers due to its wide pharmacological and biological applications over the past two decades2 but the use of benzothiazepine as corrosion inhibitors has not been reported. The aim of the present investigation is to synthesize two benzothiazepine derivatives and to study the inhibition efficiency for the corrosion of mild steel in 1M H2SO4 using weight loss, electrochemical techniques and quantum chemical calculations.
Materials and Methods
Synthesis of Benzothiazepine
The 1, 5-benzothiazepine were synthesized by the condensation reaction between o-aminothiophenol and chalkone3
 |
Scheme 1 Click here to View scheme |
The synthesized inhibitor was characterized by FTIR spectra.
 |
Figure 1: FTIR Spectrum of EPBTZ Click here to View figure |
FTIR spectra: ᶹ C=N-1600 cm-1, ᶹ C- S-654 cm-1, , C-N-1243 cm-1
Material Preparation
Mild steel specimens of dimensions 1cm X 3cm X 0.1cm with composition of 0.084%C, 0.369% Mn, 0.129% Si, 0.025%S, 0.022% Cr, 0.01%Mo, 0.015% Ni and remaining iron were used for weight loss measurements. For electrochemical measurements, mild steel rod of same composition embedded in Teflon with exposed area of 0.785cm2 was used. The specimens were polished with various grades of emery sheets, washed with double distilled water, degreased with acetone and dried. The specimens were stored in a desicator.
Weight loss measurements
The corrosion media used is 1M H2SO4 (100ml) without and with addition of the inhibitor. The pre weighed mild steel specimens in triplicate were immersed in the medium for 3 hours. The mild steel specimens were then removed, washed with double distilled water, dried and reweighed. From the initial and final weights of the specimen, the average weight loss of the triplicates was recorded. The inhibition efficiency, corrosion rate and surface coverage were calculated from the weight loss using the formula,
Efficiency of Inhibitor= [(Weight Loss without inhibitor- Weight loss with inhibitor)/Weight loss without inhibitor] X100
Corrosion rate (mpy) = [(534 X Weight loss in mgms) / (Density X Area in sq. inch X Time in hours)]
Surface coverage (ϴ) = [(Weight loss without inhibitor – Weight loss with inhibitor)/ Weight loss without inhibitor]
Where, ϴ is the surface coverage. The above procedure was repeated at different temperatures (313-333K).
Electrochemical Techniques
Electrochemical measurements were carried out with three electrode cell assembly using IVIUM compact stat with IVIUM SOFT software. The mild steel rod was used as working electrode, a platinum wire and a standard calomel electrode were used as auxiliary and reference electrodes respectively. The electrochemical impedance spectroscopic measurements were obtained in the frequency range of 10 KHz to 0.01 KHz at the open circuit potential with peak to peak amplitude of 10 mV. From the Nyquist plot the charge transfer resistance (Rct) and double layer capacitance (Cdl) were calculated.
I.E (%) = [( Rct* – Rct) / Rct*] X 100
Where, Rct and Rct* are the charge transfer resistance obtained in the absence and presence of the inhibitors.
The potentiodynamic polarization curves were obtained at a sweep rate of 1mV/s starting from-200mV to +200 mV at the open circuit potential.
I.E (%) = [(Icorr – Icorr(inh))/ Icorr] X 100
SEM Study
The surfaces of the mild steel plates after immersion in blank acid and acid containing maximum concentration of the inhibitors were examined using Medzer Biomedical research microscope (Shimadzu, Japan).
Results and Discussion
Weight loss measurements
The corrosion rate of mild steel with the addition of the benzothiazepines in 1M H2SO4, inhibition efficiency and surface coverage (θ) are presented in Table1. From the table it is clear that there is an increase in inhibition efficiency with increase in concentration. At 10 ppm the efficiency reached an optimum value. Beyond this concentration, the efficiency was found to be almost constant.
Table 1: Inhibition efficiency of various concentrations of the inhibitors for corrosion of mild steel in 1M H2SO4 obtained by weight loss measurements at room temperature.
| Inhibitor | Concentration (ppm) | Weight loss (g) | Inhibition Efficiency (%) | Degree of surface coverage(Ө) | Corrosion rate (mpy) |
| BLANK | – | 0.2656 | – | – | 17466.71 |
| EPBTZ | 2 | 0.087 | 67.24 | 0.6724 | 5721.40 |
| 4 | 0.0746 | 71.91 | 0.7191 | 4905.93 | |
| 6 | 0.0618 | 76.73 | 0.7673 | 4064.16 | |
| 8 | 0.0405 | 84.75 | 0.8475 | 2663.41 | |
| 10 | 0.0351 | 86.78 | 0.8678 | 2308.28 | |
| MPPBTZ | 2 | 0.0986 | 62.87 | 0.6287 | 6484.25 |
| 4 | 0.0920 | 65.36 | 0.6536 | 6050.21 | |
| 6 | 0.0770 | 71.00 | 0.7100 | 5063.76 | |
| 8 | 0.0700 | 73.64 | 0.7364 | 4603.42 | |
| 10 | 0.0650 | 77.22 | 0.7722 | 3978.67 |
Adsorption isotherm
The interaction between the inhibitor and mild steel surface can be studied using adsorption isotherms. The surface coverage values (θ) obtained from weight loss method and were tested by fitting to various isotherms. It was found that the best fit was obtained with Langmuir isotherm which is expressed as
C/ Ɵ = 1/Kads + C
Where C is the concentration of the inhibitor and Kads is the adsorption equilibrium constant for the adsorption desorption process. Kads represents the adsorption power of the inhibitor molecule on the surface. The linear plots(C/ Ɵ Vs C) obtained in Figure 2 with correlation coefficient R2=0.99 suggest that the adsorption of the studied inhibitors from 1M H2SO4 followed Langmuir adsorption isotherm. The intercept of the plot on the y-axis gives the value of 1/K from which K can be obtained. The positive value confirms the adsorbability of the inhibitors on the metal surface. Kads is related to ∆Gads as,
∆Gads= -RT ln (55.5 Kads)
The value 55.5 is molar concentration of water. The value of ∆Gads is found to be negative and less than – 40 KJ/mole (Table: 3) suggesting that the adsorption of the studied benzothiazepines are spontaneous and involves electrostatic interaction with the mild steel surface.
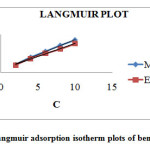 |
Figure 2: Langmuir adsorption isotherm plots of benzothiazepine Click here to View figure |
The slope, equilibrium constant and regression coefficient are given in Table 2.
Table 2: Adsorption parameters calculated from the Langmuir adsorption isotherm
| Compound | Temperture (K) | Kads | R2 | -ΔG | slope | Intercept |
| EPBTZ | 303 | 1.005 | 0.999 | 10.13 | 1.04 | 1.13 |
| MPPBTZ | 303 | 0.884 | 0.998 | 9.80 | 1.22 | 0.99 |
Effect of temperature
The effect of temperature on corrosion and inhibition efficiency of the inhibitors was studied by conducting weight loss measurements at 303-333K.The data are given in Table3.
Table 3: Inhibition efficiencies of the inhibitor for corrosion of mild steel in 1M H2SO4 obtained by weight loss measurements at higher temperatures.
| Name of the inhibitor | Temperature (K) | Weight loss(g) | Inhibition efficiency (%) | Corrosion rate(mpy) |
| Blank | 303 | 0.0885 | – | 17460.14 |
| 313 | 0.1152 | – | 22727.60 | |
| 323 | 0.1443 | – | 28468.69 | |
| 333 | 0.2486 | – | 49045.85 | |
| EPBTZ | 303 | 0.0117 | 86.77 | 2308.27 |
| 313 | 0.0206 | 76.77 | 4064.13 | |
| 323 | 0.0298 | 68.58 | 5484.61 | |
| 333 | 0.0324 | 63.38 | 6392.13 | |
| MPPBTZ | 303 | 0.0201 | 77.28 | 3965.49 |
| 313 | 0.0233 | 73.67 | 4596.81 | |
| 323 | 0.0298 | 66.32 | 5879.18 | |
| 333 | 0.0348 | 60.67 | 6865.63 |
As the temperature increases, the inhibition efficiency was found to decrease. This may be due to increased rate of desorption of the inhibitor molecules at higher temperatures. The activation energy (Ea) calculated from the slopes of the Arrhenius plot (Figure 3) of the inhibited solutions was higher than the blank acid solution suggesting strong adsorption of the molecule on steel surface4. This increases the activation energy for corrosion process. The values of thermodynamic parameters such as enthalpy and entropy of activation were calculated from the slopes and intercepts of the transition state plot (log corrosion rate/T Vs 1/T). The slope of the straight line is equal to ∆Hads /2.303R and intercept equal to log R/Nh + ∆Sads/2.303R. The values are recorded in Table 4.
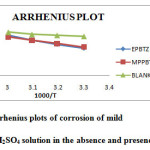 |
Figure 3: Arrhenius plots of corrosion of mild steel in 1M H2SO4 solution in the absence and presence of inhibitor Click here to View figure |
Table 4: Kinetics/Thermodynamic Parameters of mild steel corrosion in 1M H2SO4
| Name of the inhibitor | Ea (kJ) | ΔG0ads | -∆H0 kJ/mole | -∆S0 kJ/mole | |||
| 303K | 313K | 323K | 333K | ||||
| BLANK | 27.7 | – | – | – | – | 12.18 | 0.64 |
| EPBAZ | 37.59 | – 9.05 | – 7.56 | – 6.69 | -6.26 | 34.7 | 0.60 |
| MPPBTZ | 31.06 | – 7.40 | – 7.13 | – 6.42 | – 5.94 | 42.4 | 0.89 |
The negative values of ∆H suggest that the adsorption of the benzothiazepines on mild steel is an exothermic process5. The ΔGadsvalues are less than -20KJ/mol which implies physisorption of benzothiazepine on the mild steel surface involving the positively charged surface and the lone pair of electrons on heteroatoms and π electrons of the ring. ∆S values are negative suggesting that the adsorption of the inhibitor leads to an ordered state during adsorption.
Electrochemical Techniques
Electrochemical Impedance spectroscopy
The effect of EPBTZ on the impedance behavior of mild steel in 1M H2SO4 is shown as Nyquist plots. The results are analyzed with an equivalent circuit model shown in Fig4. It is apparent from the plot that increase in the concentration of the inhibitor resulted in an increase in the impedance of the interface with increase in the diameter of the semicircles. Cdl value decreased and the Rct value increased with increase in concentration. These results clearly indicate that the corrosion of mild steel in 1M H2SO4 occurs through charge transfer process6.
Table 5: AC-impedance parameters for corrosion of mild steel for selected concentrations of the inhibitor in 1M H2SO4
|
Name of the inhibitor |
Concentration (ppm) | Rct (ohm cm2) | Cdl(µF/cm2) | Inhibition efficiency (%) |
| BLANK | – | 11.06 | 27.8 | – |
| EPBTZ | 2 | 34.0 | 22.6 | 67.47 |
| 6 | 48.2 | 23.8 | 77.05 | |
| 10 | 84.2 | 21.2 | 86.86 | |
| MPPBTZ | 2 | 30.2 | 26.3 | 63.33 |
| 6 | 38.4 | 27.4 | 71.19 | |
| 10 | 47.3 | 26.7 | 76.61 |
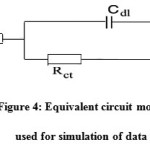 |
Figure 4: Equivalent circuit model used for simulation of dataClick here to View figure |
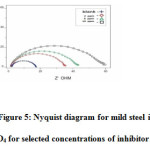 |
Figure 5: Nyquist diagram for mild steel in 1M H2SO4 for selected concentrations of inhibitor (EPBTZ)Click here to View figure |
Polarization study
Polarization experiments were carried out to study the effect of the inhibitor on the electrochemical behavior of mild steel in 1M H2SO4without and with various concentration of the benzothiazepine. Electrochemical parameters such as corrosion potential (Ecorr), corrosion current density(Icorr), cathodic and anodic Tafel slopes(bc and ba) were derived from tafel plots (Figure5) .From the plots it is evident that addition of benzothiazepine decreased the cathodiccurrent while in the anodic domain, the presence of inhibitor does not decrease the corrosion current. The inhibition efficiency was calculated using the equation,
I.E (%) = [(Icorr(Blank) – Icorr(inh))/ Icorr(Blank)] X 100
From the results it is clear that Ecorr values are shifted to less negative direction. Tafel slopes ba and bc are changed but bc is affected more. Hence the inhibitors can be regarded as mixed type but affect cathodic reaction more.
Table 6: Corrosion parameters for corrosion of mild steel with selected concentrations of the inhibitors in 1M H2SO4 by Potentiodynamic polarization method
| Name of the inhibitor | Concentration (ppm) | Tafel slopes(mV/dec) | Ecorr(mV) | Icorr(mAmp/cm2) | Inhibition efficiency (%) | |
| b a | bc | |||||
| BLANK | – | 61 | 137 | -491.0 | 414 | – |
| EPBTZ | 2 | 57 | 154 | -463.2 | 140 | 66.18 |
| 6 | 62 | 160 | -454.3 | 101 | 75.60 | |
| 10 | 65 | 167 | -452.3 | 52 | 87.43 | |
| MPPBTZ | 2 | 63 | 158 | -465.6 | 165 | 60.14 |
| 6 | 59 | 164 | -469.4 | 115 | 72.22 | |
| 10 | 64 | 170 | -471.3 | 60 | 85.50 | |
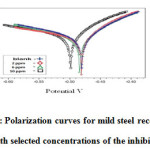 |
Figure 6: Polarization curves for mild steel recorded in 1M H2SO4 with selected concentrations of the inhibitor (EPBTZ)Click here to View figure |
SEM Study
Fig 6 and 7 show the SEM micrographs of mild steel specimen immersed in blank acid and inhibited acid (1M H2SO4 + 10 ppm). It is clear that the surface of the mild steel in presence of the inhibitor is smoother when compared to the uninhibited solution, means that the corrosion of mild steel is reduced by the adsorbed layer of the inhibitor.
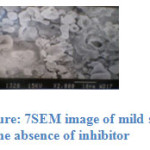 |
Figure: 7 SEM image of mild steel in the absence of inhibitor |
 |
Figure: 8 SEM image of mild steel containing 10 ppm of EPBAZClick here to View figure |
Theoretical Calculations
Quantum chemical calculations are used to correlate the molecular structure with the inhibition efficiency. Gaussian 03 program B3LYP/6-31G (d, p) was used and all calculations were carried out by complete geometry optimization. The optimized structure and frontier molecular orbital density distribution of the inhibitors are presented in Table7. The E HOMO indicates the ability of the molecule to donate electrons whereas E LUMO indicates the ability to accept electrons7-8. If the value of E HOMO is high, the molecule has a greater ability to donate electrons and greater will be the inhibition efficiency. The energy gap is an important parameter to study the adsorption of the inhibitor on the metallic surface. The inhibition efficiency of the molecule is increased with a decrease in the value of ∆E. A molecule with lower energy gap is termed as soft molecule as it is easily polarisable. The calculated quantum chemical parameters are tabulated (Table 8).
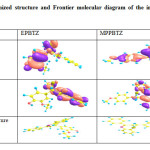 |
Table 7: Optimized structure and Frontier molecular diagram of the inhibitors EPBTZ and MPPBTZ |
Table 8: Quantum Chemical parameters for the inhibitors
| Inhibitor | EHOMO (eV) | ELUMO (eV) | ∆E (eV) | ϰ(eV) | ᶯ (eV) | σ (eV) |
| EPBTZ | -5.38 | -1.61 | 3.77 | 3.49 | 1.88 |
0.53 |
| MPPBTZ | -5.61 | -1.55 | 4.05 | 3.58 | 2.03 |
0.49 |
From the results shown, EPBTZ was found to be efficient inhibitor as it possess higher value of EHOMO (-5.38), lower value of ELUMO (-1.61) and higher value of σ (0.53). Generally it is believed that hard molecules have large energy gap whereas soft molecules have lower energy gap and are found to be more reactive9. From the above values, it is predicted that the inhibitor EPBTZ has lower energy gap (3.77 eV). It can therefore be absorbed on to the surface of the mild steel more efficiently than MPPBTZ. This may be attributed to the additional anchoring –OH group in EPBTZ.
Conclusion
- The synthesized benzothiazepine derivatives are good corrosion inhibitor for mild steel in sulphuric acid medium and act by adsorption
- The adsorption of the compounds obey Langmuir adsorption isotherm.
- Electrochemical study shows that they are mixed type but slightly cathodic.
- SEM studies confirmed the formation of a protective film of the inhibitor on the mild steel surface.
References
- Babu R. R., Thangavel K., Anti corrosion methods and materials. 2005, 52, 219.
CrossRef - Khan A. J., Baseer M.A., Orient.J.Chem. 2011, 27, 1759.
- Vutla V.R., Yejella R.P., Ramaraonadendla and Ramarao N. V., Der Pharmacia Letter, 2013, 5, 93.
- Achary G., Sachin H. P., ArthobaNaik Y., Venkatesha T. V., Mater. Chem. Phys. 2008, 107, 44.
CrossRef - Gomma M. K., Wahdan M. H.,Mater Chem. Phys. 1995, 39, 209.
CrossRef - Avci G.,Colloids and Surfaces A: Physicochem. Eng. Aspects. 2008, 317, 730.
CrossRef - Gece G., Corrosion Science. 2008, 50, 2981.
CrossRef - Zhang D. Q., Gao L. W., Zhou G. D., Corrosion science. 2004, 46, 3031.
CrossRef - Khaled K. F., ElectrochimicaActa. 2010, 55, 6523.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









