One Pot Synthesis of Formyl benzyl terpyridine – A simplified synthesis.
Anup Gurung and Sanjay Dahal*
Department of Chemistry, Sikkim Manipal Institute of Technology, Sikkim Manipal University, East Sikkim- 737136, India. Corresponding author email: dahal.sanjay@smit.smu.edu.in
DOI : http://dx.doi.org/10.13005/ojc/320253
Article Received on :
Article Accepted on :
Article Published : 16 Apr 2016
Formyl phenyl terpyridine was synthesized from methyl phenyl terpyridine using SeO2 as the oxidizing agent. SeO2 conventionally has been used to oxidize allylic and aliphatic methyl groups. The simple conversion of methyl group attached to aromatic ring appended to heterocycle in a clean one pot synthesis paves way for synthesis of similar aldehydes extendable to other classes of compound as well.
KEYWORDS:Methyl bhenyl terpyridine (4-methyl-phenyl-2,2’:6’,2’’-terpyridine); Formyl benzyl terpyridine(4-formyl-phenyl-2,2’:6’;2’’-terpyridine)”; Oxidation; Selenium dioxide
Download this article as:| Copy the following to cite this article: Gurung A, Dahal S. One Pot Synthesis of Formyl benzyl terpyridine – A simplified synthesis. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Gurung A, Dahal S. One Pot Synthesis of Formyl benzyl terpyridine – A simplified synthesis. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15397 |
Introduction
Aldehydes are the versatile reagents in the organic synthesis and especially aromatic aldehydes has been extensively used as a starting reagent for various products through various types of reactions 1-4. Synthesis of Oximes which are known for their biological activity from aldehydes has been reported in the literature5. Recently the synthesis of aryl amines from aldehydes which has pharmaceutical importance has been reported using Zn(BH4)2/ MgBr2 6. Varieties of oxidizing agents are available for oxidation of aliphatic as well as aromatic alkyl groups which gets converted to carboxylic group in majority of the reactions 7.Mild oxidizing agents has been used to oxidize methyl group to alcohol. Variety of reagents convert methyl group to aldehyde. Methods like Etard reaction, chromyl chloride oxidation and oxidation with PCC involve multi step reactions for conversion 8-11. Cerium and Iridium catalyzed reaction can oxidize methyl group attached to aromatic group like benzene 12-15 but is not successful in oxidizing methyl groups attached to heterocyclic rings. Selenium dioxide is a versatile reagent for oxidizing methyl groups directly attached to heterocyclic ring like 3-methylbipyridine, 4-methylphenantrholine, 2,5-dimethylpyridine16.Heterocyclic aldehydes are best known for their versatility in the synthesis of biologically active molecules. But the problem arises when there is a aromatic ring like phenyl ring present in between heterocycle and the methyl group. Almost all the reported methods failed to oxidise such methyl groups. Schubert et. al reported the Oxidation of such methyl group to aldehyde in a multistep process 17,18. SeO2 was found to be unsuccessful in oxidation of methyl group of nitro toluene and even that of 2,4,6-trinitrotoluene, however on conversion to phenyl bromides conversion to aldehyde was found to be feasible19. Here we report the simplified synthesis of aldehyde (FPT) in one pot using SeO2 in 1,4-dioxane paving the way for simplified oxidation of such class of compounds to aldehydes.
Experimental
Methyl phenyl terpyridine [1] (MPT)
was prepared using Krohnke methodology by condensation of 2-acetyl pyridine and p-tolualdehyde in 60% yield. 1.65 g (41 mmol) of crushed NaOH was added to the 30ml of Polyethylene Glycol (PEG-300) with constant stirring at room temperature. 5 g (41.6 mmol) of 2-acetyl pyridine was added and after 10 minutes 2.472 g (20.6 mmol) of p- tolualdehyde was added with continuous stirring. The colorless solution turned to light yellow. After two h the color changed to orange and then to pink. 25 ml of liquid NH3 (18N) was added. The white precipitate formed was washed with water and recrystallized from ethanol.
Yield %- 60%, HNMR-(CDCl3, 400 MHz, ppm) 2.4 (s, 3 H) δ 7.49 (mc,2 H), 8.01 (mc, 2 H), 8.15 (mc, 4H), 8.72 (d,2 H), 8.80 (d, 2 H), 8.98 (s, 2 H),.5, UV-Vis- 281 nm.
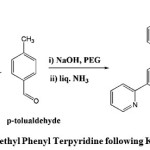 |
Figure 1: Synthesis of Methyl Phenyl Terpyridine following Krohnke Methodology. |
Formyl phenyl terpyridine[2] (FPT)
1 g (9.0 mmol) of SeO2 and 0.1 ml (5.55 mmol) of H2O was added to 700 (2.16 mmol) mg of MPT in 10 ml of 1,4-dioxane. The mixture was heated at 200oC in autoclave for 2 hours. The steel bomb was taken out and cooled to room temperature. Additional 1 g of SeO2 and 0.5 ml of H2O was added and the steel bomb was heated in autoclave for 6 hrs. The steel bomb was cooled to room temperature and the pale yellow colored product formed was dried on water bath. The product was purified by column chromatography using CHCl3 and 9:1 CHCl3: MeOH mixture as the eluent.
Yield – 20%, 1HNMR (CDCl3, 400 MHz, ppm) δ 7.39 (mc,2 H), 7.91 (mc, 2 H), 8.05 (mc, 4H), 8.69 (d, 2 H), 8.75 (d, 2 H), 8.78 (s, 2 H), 10.12 (s, 1 H).5, UV-Vis, 283 nm.
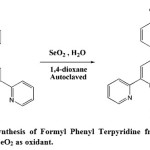 |
Figure 2: Autoclave synthesis of Formyl Phenyl Terpyridine from Methyl Phenyl Terpyridine using SeO2 as oxidant. Click here to View figure |
Table 1: Standardization of the synthesis of Formyl Phenyl Terpyridine.
| MPTmmols | SeO2mmols | 1,4-Dioxaneml | H2Ommols | Temp.
|
Time h | Yield % |
| 6.31 | 9.00 | 35 | 11.1 | 120 | 12 | 0 |
| 0.30 | 1.15 | 10 | 5.55 | 125 | 12 | 0 |
| 0.30 | 1.15 +* 1.15 | 10 | 5.55+*5.55 | 125 | 2 +* 8 | 10 |
| 0.30 | 1.80 +*1.80 | 16 | 2.77+*2.77 | 150 | 2 +*10 | 9 |
| 2.16 | 4.50+*4.50 | 10 | 5.55+*5.55 | 200 | 2+*6 | 20 |
*Time after addition of additional amount of SeO2 and H20.
Result and Discussion
Several reactions were carried out using different amount of SeO2 to obtain maximum conversion. As per the Table 1. large scale synthesis of aldehyde using higher concentration failed to yield any product even after keeping the reaction mixture at 120OC for 12 hrs. Even in low concentration the reaction failed and no product was detected. Upon slight modification of the reaction condition i.e. changing from one time addition to two time addition of oxidizing agents after an interval resulted in formation of desired product. It can be inferred with ample support from literature that in the first step of reaction the methyl group gets converted to alcohol group which in next step gets converted to the desired formyl group. Molar ratio of SeO2:MPT:H2O in proportion of 3 : 1 : 5 gave the best yield. Though small amount of unreacted MPT was detected it was reused for further reaction. The study also suggests that at the optimal temperature of 200OC, the yield is best and the reaction time required for second step of reaction could be reduced to 6hrs . The method opens up the new route for the oxidation of tolyl group attached to heterocycle to aldehyde in one pot synthesis which otherwise is not possible in normal conditions. Though the conversion was standardized with only one heterocycle it can be easily extended to other heterocycles. 1,4-dioxane was chosen on the basis of solublity of the initial/ reactants.
The aldehyde prepared has been successfully used for the synthesis of porphyrin as revealed by UV-vis spectroscopy and detailed characterization of which is under progress.
Acknowledgement
The authors would like to thanks the DST, Gov. of India for financing the research with Grant No. DST:SR/S1/IC-08/2006.
References
- Aspinall, H. C; Greeves, N; Valla, C., Org. Lett., 2005, 7, 1919-1922;
CrossRef - Duan, X.-F. ; Feng, J.-X. ; Zi, G.-F; Zhang, Z.-B, Synthesis, 2009,2, 277-282;
CrossRef - Takenaka, N.; Xia, G; Yamamoto, H, J. Am. Chem. Soc., 2004, 126, 13198-13199
CrossRef - Corey, E. J.; Gilman, N.W.; Ganem, B., E. J. Am. Chem. Soc. 1968; 90, 5616 – 5617
CrossRef - Ghozlojeh, N. ; Setamidideh, D., Orient. J. Chem., 2015, 31, 1823-1825
CrossRef - Mahmoudi, M; D, Orient. J. Chem., 2015, 31, 1215-1218
CrossRef - Dash S.; Patel S.; Mishra, B. K., Tetrahedron, 2008, 65, 724
- Ho,T. L.,Synthesis, 1972,7, 347-354
- Hwu,J.R.;King,K.Y,Current Science, 2001, 81, ,1043-1053
- Wheeler,O.H.,Can. J. Chem. , 1958,36 , 949-951
CrossRef - Ratcliffe, R. W. Org. Synth.;, 1988, 6 , 373-375
- Ho, T.L.; Mijs,W.J; de Jonge C.R.H ,Organic Synthesis by Oxidation With Metal Compounds, Plenum, New York, 1986, 569–631.
CrossRef - Nair, V.; Balagopal, L.; Rajan, R. Mathew, J.; Accounts Chem. Res.,2004,37, 21–30.
CrossRef - Demappa, M.T., J. Appl. Polym. Sci.,2006, 103,3498–3505.
CrossRef - Jiang, B. ; Feng, Y. ; Ison, E.A. , J. Am. Chem. Soc. ,2008, 130, 14462–14464
CrossRef - Trump, E. L.; Zhou M.X, Transactions of the Kansas Academy of Science,1903, 96, 167-180
CrossRef - Winter, A.; Egbe, D. A. M.; Schubert U. S., Organic Letters, 2007, 9,2345-2348 ·
CrossRef - Collin, J.-P.; Heitz,V.; Sauvage, J.-P. Tetrahedron Lett. 1991, 32, 5977–5980.
CrossRef - Fischer, C. H. Auration of Aromatic Nitriles, 1934,56, 2056-2057.

This work is licensed under a Creative Commons Attribution 4.0 International License.









