Kinetics and mechanisms of the Diels-Alder reaction of 9-bromomethyl Anthracene with Citraconic anhydride: A DFT study
Atiye Bazian, S. Ali Beyramabadi*, Abolghasem Davoodnia , Mehdi Pordel and Mohammad Reza Bozorgmehr
Department of Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran.
Corresponding author email: beyramabadi@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320239
Article Received on :
Article Accepted on :
Article Published : 16 Apr 2016
In this work, the kinetics and mechanism of the Diels-Alder cycloaddition reaction between the 9-bromomethyl antracene and Citraconic anhydride dienophile have been investigated theoretically in the toluene solution. All of the calculations have been performed by using the valuable DFT methods and PCM model.The reaction can be progressed via two different pathways, I and II. In the structure of the reactants of the pathways I and II, the 9-bromomethyl antracene and Citraconic anhydride species have different orientations. Both of the pathways have been investigated in details. The obtained DFT-results such as prediction of the major product of the reaction are in agreement with the experimental results, confirming validity of the DFT-proposed mechanism.
KEYWORDS:Diels-Alder; Regioisomer; Cycloaddition; DFT; PCM; Kinetics; Mechanism
Download this article as:| Copy the following to cite this article: Bazian A, Beyramabadi S. A, Davoodnia A, Pordel M, Bozorgmehr M. R. Kinetics and mechanisms of the Diels-Alder reaction of 9-bromomethyl Anthracene with Citraconic anhydride: A DFT study. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Bazian A, Beyramabadi S. A, Davoodnia A, Pordel M, Bozorgmehr M. R. Kinetics and mechanisms of the Diels-Alder reaction of 9-bromomethyl Anthracene with Citraconic anhydride: A DFT study. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15382 |
Introduction
The Diels-Alder pericyclic reaction [1],which is one of the most important synthetic reactions, has been illustrated to be a powerful synthetic method for multiple carbon-carbon bond formation in a regioselective manner. This has been proved by its various application in synthesys [2-5].
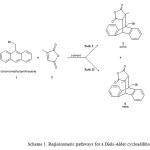
The usefulness of the Diels-Alder reactions arises from their versatility and from their remarkable regioselectivity. The interaction between unsymmetrical reagents (dienes or dienophiles) in Diels-Alder reactions can give two regioisomers depending on the relative position of the substituent in the cycloadduct. For a 9-bromomethyl anthracene diene, a head-to-head (ortho) or a head-to-tail (meta) cycloaddition is possible, where the ortho isomer is preferred(see Scheme 1).
 |
Scheme 1: Regioisomeric pathways for a Diels-Alder cycloaddition Click here to View scheme |
Some theoretical reports have been published on the kinetics and mechanism of the Diels-Alder cycloaddition reaction[6-9].Khanet al. [10] report the Diels-Alder cycloaddition reaction of the 9-substituted anthracene, species 1, as diene with Citraconic anhydride dienophilespecies 2, which gives the 3 and 4 regioisomers (Scheme 1).The 9-bromomethyl anthracene reacts with the unsymmetrical dienophile species 2 in two competitive pathways, I and II pathways, which result in formation of two regioisomeres, the 3(ortho) and the 4(meta) regioisomers, respectively. However, in most cases the ortho adduct has been found to be the major product.
We became interested in the regioselectivity of the 9-bromomethyl anthracene 1 towards species 2. In order to give a deeper insight to this reaction, we have investigated the kinetic and mechanism of this cycloaddition reaction in details, using Density Functional Theory (DFT). Both of the pathways, I and II, have been considered.
Computational Details
Herein, all of the calculations have been performed by using the B3LYP [11] hybrid functional as implemented in the Gaussian 03 program package [12]. The 6-311++G(d,p) basis set was employed.
All degrees of freedom were optimized for all geometries. The obtained transition states (TSs) were confirmed to have only one imaginary frequency of the Hessian, while the reactants, products and intermediates didn’t show any imaginary frequency.
Obviously, the solvent plays an important role in chemical reactions. Here, the solute-solvent interactions have been considered using one of the self-consistent reaction field methods, i.e., the sophisticated Polarizable Continuum Model (PCM) [13]. The solvent is chloroform. In both of the gas phase and PCM model, the zero-point-energy (ZPE) corrections were made to obtain energies. The employed methods are widely applicable in theoretical investigation of the chemical reactions [6-9, 14-18].
Results and Discussion
Based on the Scheme 1, the cycloaddition reaction of the 9- bromomethyl anthracene (species 1), with theC=C double bond of the Citraconic anhydride (2), produces the 3 and 4 regioisomers [10].Mechanism of the reaction involves two different pathways, I and II.Herein, we report a detailed-DFT investigation on the two pathways of the reaction mechanism. Finally, the DFT results of the two pathways I and II are compared both with each other. The proposed mechanism will be reviewed step by step in the following sections.
Both of the pathways (I and II)involve one step. In both of the pathways, the reactant involves species 1 and 2 in presence of each other. However, the species 1 and 2 have roughly inverse orientation in the reactant structure for each of the pathways I and II. Reactant of the pathways I and II is named as 1+2 and 1+2meta, respectively. The species 3 is the main product of the reaction, in which the C9 and C10 atoms are bonded to the C15 and C11 atoms, respectively. But, in the structure of the species 4, the C9 and C10 atoms are bonded to the C11 and C15 atoms, respectively, which is the inverse of that for the species 3.In following, this mechanism has been investigated in details.
The pathway I mechanism
In the pathway I, the reactant of the reaction involves the species 1 and 2in presence of each other. Optimized geometries of the reactant (1+2), transition state (TS1) and product (species 3) of the pathway I are shown in Figs. 1, 2 and 3, respectively. The C9 and C10 atoms of the species 1 bond covalently to the C15 and C11 atoms of the species 2, respectively, to produce species 3. In the optimized geometry of the reactant (Fig. 1), the C9-C15 and C10-C11 distances are 6.02 and 3.90Å, respectively, which reduce to 1.62 and 1.57Å in the optimized geometry of the product, 3, respectively. These distances are 2.56 and 2.00Å in the optimized geometry of the TS1, respectively, showing formation of a bicyclic adduct3. These data show suitability of the obtained geometry for the TS1 as the transition state of the pathway I.
Going from reactants to the species 3, the C9-C15 and C10-C11 bond lengths reduce, while the C9-C16, C10-C17 and C11-C15 bond lengths increase from 1.42, 1.40 and 1.33Å in the reactant to 1.54, 1.51 and 1.55 Å in the structure of product, 3. The calculated C17-C16-C9-C19 dihedral angle is -2.72, -29.78 and -50.50° in the optimized geometries of the reactant, TS1 and product, respectively. In the structure of the TS1, the C9 and C10 atoms exit from the plane of anthracene, providing suitable condition for formation of the C9-C15 and C10-C11 bonds.The calculated-activation energy (Ea) for the pathway I is 113.78 kJ.mol-1 in the toluene solution.
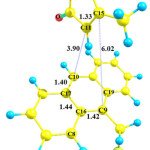 |
Figure 1: The optimized geometries of the reactant of the pathway I (species 1+2).
|
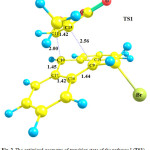 |
Figure 2: The optimized geometry of transition state of the pathway I (TS1). |
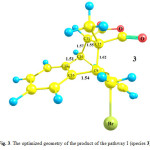 |
Figure 3: The optimized geometry of the product of the pathway I (species 3).
|
The pathway I mechanism
Fig.4Shows optimized geometry for the reactant of the pathway II (species 1+2meta), which includes the species 1 in presence of the species 2. Their orientation is suitable for formation of the meta product, species4. Optimized geometries of the transition state (TS2) and the product (species 4) of this pathway are shown in Figs. 5 and 6, respectively. Going from the reactants to the product (4), some of structural parameters have been changed, the most important of which is the C17-C16-C9-C19 dihedral angle. This angle is -2.39, -32.17 and -50.47° in the optimized geometries of the reactant, TS2 and product, respectively. The C9 and C10 atoms exit from the plane of anthracene, providing the necessary conditions for formation of the C9-C11 and C10-C15 bonds.
Also, the C9-C11 and C10-C15 bonds lengths undergo essential changes. These distances are long in the structure of the reactant, by 4.12 and 5.40 Å, respectively, which decrease to 2.33 and 2.09 Åin the optimized geometry of the TS2, respectively. In the optimized geometry of the species 4 as the main product, these bond lengths are 1.60 and 1.58Å, respectively.In addition, the C9-C16,C10-C17 and C11-C15 bonds elongate from 1.42, 1.40and 1.33 Å for the reactant to 1.54, 1.51 and 1.55Å for the product, respectively. In the optimized geometry of the TS2, these bond lengths are 1.45, 1.44 and 1.42 Å, respectively. The PCM-calculated Ea of the pathway II is 117.01 kJ.mol-1 in the toluene solution.
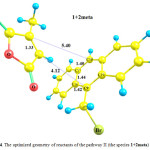 |
Figure 4: The optimized geometry of reactants of the pathway II (the species 1+2meta)
|
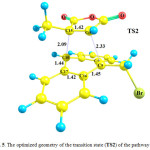 |
Figure 5: The optimized geometry of the transition state (TS2) of the pathway II.
|
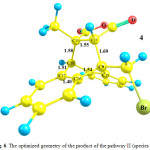 |
Figure 6: The optimized geometry of the product of the pathway II (species 4).
|
According to the energy values in Table 1, the Eas of the pathway I and II in toluene solution are 113.78 and 117.01 kJ. mol-1, respectively. Thus, the energy barrier of the pathway II is higher than the pathway I.The reaction profile for the both pathways of the mechanism has been shown in diagram 1.
 |
Diagram 1: The reaction profiles of the pathways I and II ofthe investigated mechanism. Click here to View Diagram |
The reaction mechanism could progress via two different pathways, I and II, which product species 3 and 4, respectively. In toluene solution, the energy barrier of the pathway II is higher than the pathway I by 3.23 kJ.mol-1. Since, progress of the reaction in the pathway I is more favorable than the pathway II. The product of the pathway I, species 3 (ortho), is predicted to be major product of the Diels-Alder cycloaddition reaction of the 9-bromomethyl antracene and citraconic anhydride dienophile. These results are in good agreement with the experimental results, confirming suitability of the proposed mechanism for the Diels-Alder cycloaddition reaction between 9-Bromomethyl Antracene and unsymmetricaldipolarophile.
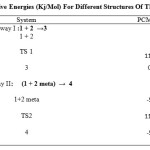 |
Table 1: Relative Energies (Kj/Mol) For Different Structures Of The Mechanism Click here to View table |
Conclusion
Herein, the kinetics and mechanism of the Diels-Alder cycloaddition reaction between the 9-bromomethyl antracene and Citraconic anhydride dienophile have been investigated in detail in the toluene solution, using the DFT methods and PCM model.
The reaction can be progressed through two different pathways, I and II. The species 1 reacts with the species 2 in two different orientations to produce the species 3 and 4 as the final products in the pathways I and II, respectively. In both of the pathways, reaction occurs in one step.
It’s worth noting that the pathway II, producing of the species 4, has higher energy barrier than the pathway I, which results to production of the species 3. Difference between their Eais 3.23 kJ.mol-1 in the toluene solution. Therefore, the reaction mainly progresses via the pathway I.The species 3, is the kinetically more favorable product than the species 4 as the product of the pathway II.
The obtained results of the DFT investigations such as the major product of the reaction are in consistent with the experimental results. It can be considered verification of the proposed mechanism.
References
- Diels O.;. Alder K; Ann. Chem. 1928,98, 460.
- Carruthers W.; Cycloaadditions reactions in organic synthesys ; Pergamon: Oxford , 1990.
- Nicolaou K. C.;. Snyder S. A; T. Montagnon; G. Vassilikogiannakis; Angew. Chem. Int.Ed. 2002,41, 1668.
CrossRef - Williams R. M.; Stocking E. M.; Angew. Chem. Int. Ed. 2003, 42, 3078
CrossRef - Futatsugi K.; Yamamoto H.; Angew. Chem. Int. Ed. 44, 1484 (2005).
CrossRef - Domingo L. R.;. Picher M. T;. Andres J;. Safont V. S; J. Org. Chem. 1997, 62, 1775
CrossRef - Noorizadeh S;. Maihami H; Theochem,2006, 763, 133
CrossRef - Beno; B. R. Houk K. N.; Singleton D. A.; J. Am. Chem. Soc. 1996, 118, 9984.
- Domingo L. R.;. Aurell M. J; Perez P.;. Contreras R; J. Phys. Chem. 2002,106, 6871
CrossRef - Khan R.;. Singh T. P; Singh M. D.; Synlett,2014, 25, 0696
- Lee C., Yang W., Parr R.G., Phys Rev B 1988,37, 785
CrossRef - Frisch M.J., et al., Gaussian 03, Revision C.02; Gaussian, Inc.: Pittsburgh, PA 2003.
- Tomasi J., Cammi R., J. Comput. Chem. 1995, 16, 1449
CrossRef - Beyramabadi S.A., Eshtiagh-Hosseini H., Housaindokht M.R., Morsali A., Organometallics, 2008, 27, 72.
CrossRef - Wang H., Res. Chem. Intermediat. 2012, 38, 2175
CrossRef - Vahidi S. H., Morsali A., Beyramabadi S. A., Comput. Theo. Chem. 2012, 994, 41.
CrossRef - Najafi Ardabili M., Morsali A., Beyramabadi S.A., Chegini H., Gharib A., Res. Chem. Intermediat. 2015, 41, 5389
CrossRef - Eshtiagh-osseini H.,. Beyramabadi S.A,. Morsali A, Mirzaei M., Chegini H., Elahi M., Naseri M.A., J. Mol. Struct. 2014,. 1072, 187
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









