Effect of NiW Modified HZSM-5 and HY Zeolites on Hydrocracking Conversion of Crude Palm Oil to Liquid Hydrocarbons
Maliwan Subsadsana and Chalerm Ruangviriyachai*
Materials Chemistry Research Center,Department of Chemistry and Center of Excellence for Innovation in Chemistry (PERCH-CIC), Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand. Corresponding author email: chal_ru@kku.ac.th
DOI : http://dx.doi.org/10.13005/ojc/320208
Article Received on :
Article Accepted on :
Article Published : 15 Apr 2016
The catalytic conversion of crude palm oil over HZSM-5 and HY zeolites modified with NiW as catalysts in the hydrocracking process was investigated. These zeolites supported by NiW catalysts were prepared employing the impregnation technique. NiW was added to the zeolites in order to induce bi-functional properties (both acid and metal sites) in the catalysts. Subsequently, the catalysts were characterized by X-ray diffraction spectrometry (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), ammonia temperature programmed desorption (NH3-TPD) andnitrogen adsorption-desorption isotherms analysis. The catalytic activity of prepared catalysts was evaluated through the conversion of crude palm oil to biofuels. These results indicate that the incorporation of NiW over HZSM-5 and HY zeolites improves the conversion efficiency and enhances the yield of biofuel (gasoline, kerosene, and diesel), possibly due to NiW promote of hydrogenation and dehydrogenation reaction.
KEYWORDS:NiW-HZSM-5 zeolite; NiW-HY zeolite; Hydrocracking; Crude palm oil
Download this article as:| Copy the following to cite this article: Subsadsana M, Ruangviriyachai C. Effect of NiW Modified HZSM-5 and HY Zeolites on Hydrocracking Conversion of Crude Palm Oil to Liquid Hydrocarbons. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Subsadsana M, Ruangviriyachai C. Effect of NiW Modified HZSM-5 and HY Zeolites on Hydrocracking Conversion of Crude Palm Oil to Liquid Hydrocarbons. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15374 |
Introduction
Among all of the vegetable oils, palm oil is one of the vegetable oil that has the greatest possibility to be used in biofuel production since it is one of the most produced vegetable oil in the world. Several researches have been studied in order to efficiently convert this vegetable oil into biofuels such as gasoline and diesel fractions byhydrocracking process1-2. Generally, this process involves the utilization of commercially available catalysts and is operated at temperature above 450 °C. Hence, the development of new catalysts, with higher activity under lower temperature conditions, is needed to improve the product yield of palm oil conversion3-4.
Zeolite has been widely used as a suitable acidity component in the hydrocracking process due to its appropriate pore structure, thermal stability, and acidity5-6. It has been reported that the use of strongly acidic catalysts such as H-zeolite can overcome the chemical inertness of the starting alkanes by performing the process at high temperatures7-8. Various types of zeolite catalysts were reported in catalytic cracking for the production of biofuels from vegetable oil. They possess a great potential as solid acid cracking catalyst due to its structure, acidity and high selectivity for the production of biofuels from vegetable oils9-11. Their catalytic activity involves both hydrogenation and non-hydrogenation processes. The first is a conversion of the triglyceride content in vegetable oil into biofuels, which have lower cyclic hydrocarbon and aromatic contents compared with the conventional ones, while the latter is the dehydration and decarboxylation of biomass oil into gaseous and liquid hydrocarbons, coke, and water12-14.
The modification of zeolite with metals has been widely studied since this incorporation can increase the catalytic cracking for the conversion of vegetable oil. Recently, various catalysts such as silica-alumina, USY, b and HY-zeolites modified with bimetallic nano particles such as NiW, NiMo or CoMo has been reported15-16. This combination of metals presents the bi-functional properties, both acid and metal sites,which results in an enhanced efficiency in the hydrocracking conversion of vegetable oils into high quality biofuels 17-20.
In this work, the incorporation of bimetallic NiW over the HZSM-5 and HY zeolites was studied. Zeolites were first prepared to the H-form by ion exchange process. The HZSM-5 and HY zeolites were modified with bimetallic NiW by impregnation method. The catalytic activity of NiW modified HZSM-5 and HY zeolite was evaluated through the hydrocracking conversion of crude palm oil. It can be expected that NiW-HZSM-5 and NiW-HY catalyst could show high activity in crude palm oil hydrocracking process.
Experimental
Materials and Chemicals
Nickel nitrate hexahydrate [Ni(NO3)2.6H2O] and ammonium metatungstate [(NH4)6W12O40] from Sigma-Aldrich (USA) were utilized as a source of Ni and W, respectively. Ammonium sulphate [(NH4)2SO4] was obtained from Labs can(Thailand) for ion exchange. The NaZSM-5 zeolite with SiO2/Al2O3 ratio 50 was purchased from thezeolyst international, USA, and ammonium Y zeolite obtained from Sigma-Aldrich, USA, was used as the reference catalyst. Crude palm oil was supplied by Ubon Ratchathani Palm Oil Co., Ltd., Ubon Ratchathani Province, Thailand,and was further used in the hydrocracking process.
Catalysts
Firstly, the NaZSM-5 zeolite was treated in ammonia ion exchange process. An ion exchange process was carried out with a 1 M (NH4)2SO4 solution at 90 °C for 2 hto give the H-form products (HZSM-5 zeolite). After that, this zeolite was calcined at 550 °C for 3 h prior to use. In NH4Y zeolite, ammonia ion was thermally eliminated at 110 °C for 4 h and further calcined at 550 °C for3 h. After that, NiW catalysts were deposited on the surface of the zeolite supports by the conventional impregnation technique. A support prepared above was immersed to a mixture solution of Ni(NO3)2.6H2O and (NH4)6W12O40 for 1 hr. Each catalyst supported contains 8 wt.% W and 4 wt.% Ni. After impregnation, the catalyst was dried at 110 °C for 5 h, and then calcined at 550 °C for 3 h.
Characterization
The physicochemical properties of the prepared catalysts were characterized by means of various conventional techniques. X-ray diffraction patterns were obtained using a Shimadzu-6000 diffractometer with Cu Kα radiation at 40 mA and 40 kV. The step size of 0.02 and a step time of 0.5 s for the range of 5 to 80° were employed. The total amount of Ni and W in the impregnated catalyst was determined by PANalytical PW-2404 X-ray fluorescence spectroscopy (XRF).The morphologies were examined using JSM-5410LV scanning electron microscope (SEM). Transmission electron microscopy (TEM) micrographs were recorded with a JEOL 2010 F electron microscope operating at 200 kV. Prior to the observation, the catalysts were dispersed in ethanol solution, stirred in an ultrasonic bath and deposited on a copper grid. The surface area and porous properties of catalysts before and after modification with NiW were studied using nitrogen adsorption-desorption isotherms recorded on a Quanta chrome Auto sorb Automated Gas Sorption analyzer at 77 K. The catalysts were degassed in vacuum for 3h at 300 °C. The surface area was calculated by the brunauer-emmett-teller (BET) isothermal equation, based on p/po data in the range of 0.0-1.0. The pore volume was obtained from the t-plot method. The acid properties were measured by ammonia temperature programmed desorption (TPD) in a Quantachrome Chem BET 3000 with a thermal conductivity detector (TCD). 80 mg sample was placed in a quartz tubular reactor and pretreated at 600 °C with a N2 flow of 30 mL/min for 1 h and then cooled to 100 °C. Ammonia diluted with Ar (5% v/v NH3) was then introduced at a flow rate of 30 mL/min for 1 h at 100 °C and then a He stream was fed in until TCD signal was constant. NH3-TPD was performed with the reactor temperature at a ramp rate of 10 °C/min. from 100 °C to 700 °C.
Catalytic Hydrocracking
In order to evaluate the activity of the prepared catalyst, the catalytic conversion of crude palm oil (500 cm3) was performed in batch reactor with a volume of 1 L. 5 g catalyst was fed into the reactor. The mixture was automatically and continuously stirred at 500 rpm. Then, the reactor was heated electrically with a control of a thermocouple. Initially, a reactor was purged with N2 gas for 30 min. to completely eliminate O2 gas. Then, the reactor was flown with H2 gas at a flow rate of 500 cm3min-1and 3.0 MPa pressure for 30 minute. Thereafter, the catalytic runs were performed by heating the reactor up to 400 °C and then hold for 120 min. of reaction time (pressure up to 8.0 MPa). After the complete catalytic reaction, the reactor was stopped and allowed to cool down to the ambient temperature. Furthermore, the liquid product from hydrocracking process was distilled, separated using a distillation funnel at 150-350 oC, and collected with a glass vial.
Results and Discussion
The surface topography, crystal structure, chemical component, surface area, and pore volume of NiW-HZSM-5 and NiW-HY catalysts were characterized by XRD, SEM, TEM, NH3-TPD and N2 adsorption-desorption measurement, in comparison with the corresponding HZSM-5 and HY zeolites.
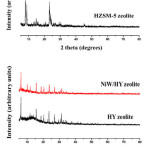 |
Figure: 1 XRD patterns of HZSM-5 and HY zeolites before and after modification with NiW |
XRD patterns of HZSM-5, HY, and impregnated catalysts are shown in Fig. 1. The results showed the similar characteristic peaks, indicating that the impregnation of HZSM-5 and HY zeolites with NiW did not affect the crystallinity of zeolites. Additionally, the disappearance of Ni and W peaks was observed, possibly due to the dispersion of metals over the external surface of HZSM-5 and HY zeolites.
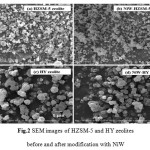 |
Figure 2: SEM images of HZSM-5 and HY zeolites before and after modification with NiW |
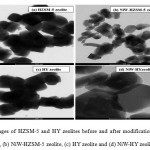 |
Figure 3: TEM images of HZSM-5 and HY zeolites before and after modification with NiW: (a) HZSM-5 zeolite, (b) NiW-HZSM-5 zeolite, (c) HY zeolite and (d) NiW-HY zeolite, respectively Click here to View figure |
As shown in Fig. 2, SEM images illustrated that HZSM-5 and HY zeolites are easily aggregated to form nanoparticles. Therefore, the modification of zeolites with NiW did not change the physical morphologies of HZSM-5 and HY zeolites. Fig. 3 illustrates TEM images of the catalysts before and after the incorporation with NiW. TEM images show the presence of relatively large NiW over the external surface of HZSM-5 zeolite crystals, as can be seen in Fig.3b. In contrast, the NiW located mainly within the HY zeolite channels rather than on the external surface of the zeolite nanocrystals, as shown in Fig. 3d. Therefore, HZSM-5 zeolite presents a much higher dispersion of metal than HY zeolite.
Table 1: Metal Content, BET Surface Area And Pore Volume Of Catalyst Before And After Modification With Niw
|
Catalyst |
Metal content (wt%) |
Before modification |
After modification |
|||||
|
Ni |
W |
Surface area (m2g-1) |
Pore volume (cm3g-1) |
Surface area (m2g-1) |
Pore volume (cm3g-1) |
|||
| HZSM-5 |
3.11 |
7.33 |
345.9 |
0.305 |
253.4 |
0.196 |
||
| HY |
3.16 |
7.42 |
249.8 |
0.212 |
221.7 |
0.119 |
||
The textural properties of the catalysts were determined using the N2 adsorption isotherms and the surface area was calculated by the BET method. Table 1 shows the difference in textural properties of catalysts before and after modification with NiW. When adding NiW into the zeolite, it was found that a more pronounced decrease in the micropore volume takes place, resulting from a partial blockage of the zeolite channels by metal particles. The results are in agreement with the reduction of the BET surface area for the NiW modified zeolites.
Table 2 shows the effect of the catalysts with different type zeolite and doping metals on the biofuels yield of the crude palm oil reaction. It was found that the hydrocracking reaction products increased significantly when Ni and W were incorporated into the catalyst, possibly due to their role as promoters in hydrogenation and dehydrogenation and aromatic hydrocarbons reaction. Indeed, most of metals are dispersed on the outer surface and the metallic diffusion and its sintering throughout the framework affect the catalyst. It can be seen that the gasoline yield over the NiW–HY zeolite catalyst was higher, compared to that over the NiW–HZSM-5 zeolite catalyst, while the kerosene yield over theNiW–HZSM-5 zeolite catalyst was higher , compared to that over the NiW–HY zeolite catalyst. The results indicated that the catalysts doped with NiW had better hydrocracking performance, compared to the catalyst without metal modification. It can also be observed that the NiW–HY zeolite catalyst had higher acid amount, when compared to the NiW–HZSM-5 zeolite. The results imply that catalyst with higher acid amount shows better activity. Moreover, the NiW-HZSM-5 and NiW-HY cataysts exhibit enhanced production of liquid biofuels with a significant increase in gasoline, kerosene and diesel fractions.
Table 2: The effect of The catalysts with different type zeolite doping metals on biofuels of crude palm oil reaction (n=3)
|
Catalyst |
Acid amount (10-4mol/g) |
Conversion (%) |
Yield (%) |
||
|
Gasoline (150-200 °C) |
Kerosene (250-300 °C) |
Diesel 350 °C |
|||
|
HZSM-5 |
0.42 |
37.20 |
6.30 ± 0.2 |
14.10 ± 0.3 |
16.80 ± 0.5 |
|
HY |
0.76 |
38.57 |
8.65 ± 0.8 |
13.30 ± 0.2 |
16.62 ± 0.2 |
|
NiW-HZSM-5 |
0.33 |
48.18 |
10.40 ± 0.1 |
19.56 ± 0.4 |
18.22 ± 0.6 |
|
NiW-HY |
0.63 |
50.38 |
13.88 ± 0.2 |
18.20 ± 0.5 |
18.30 ± 0.3 |
Conclusions
The conversion of crude palm oil into liquid hydrocarbons over bimetallic NiW -HZSM-5 and NiW-HY catalysts was studied. NiW was deposited on the surface of zeolites by impregnation method. The incorporation of NiW into the HZSM-5 and HY zeolites causes an important change in textural properties, which has an influence on the catalytic conversion. It was found that the conversion efficiency over NiW- HZSM-5 and NiW-HY was higher than that of HZSM-5 and NiW-HY catalysts, indicating that an incorporation of NiW in zeolites improves their catalytic activity. Hence, bi-functional materials, based on W and Ni loaded over crystalline HZSM-5 and HY zeolites, can be considered interesting catalysts for the conversion of crude palm oil into biofuels.
Acknowledgements
This work is supported by Nakho nRatchasima Rajabhat University for financial support and Materials Chemistry Research Center, Department of Chemistry Faculty of Science, Khon Kaen University, Thailand, for research facility and grant. In addition, National Research University project of Thailand, the Biofuel Cluster of Khon Kaen University, Thailand, are also gratefully acknowledged for the chemicals.
References
- N. Taufiqurrahmi, A.R. Mohamed, S. Bhatia. Chem. Eng. J., 2010; 163, 413-421.
CrossRef - Y.S. Ooi, A.R. Zakaria, R. Mohamed, S. Bhatia. Appl. Catal. A: Gen.,2004; 274, 15-23.
CrossRef - Y. Zhao, X. Lin, D. Li. Chem. Eng. Technol., 2015; 38, 297-303.
CrossRef - N. Chang, Z. Gu, Z. Wang, Z. Liu, X. Hou, J. Wang. J. Porous Mater.,2011; 18, 589-596.
CrossRef - Q. Jian, Z. Tianbo, X. Xin, L. Fengyan, S. Guida. China. Pet. Process. Pe.,2010; 12, 17-22.
- W. Guang, W. Wei, W. Xin, Z. Wang, W. Wenjing, L. Cheng. MicroporousMesoporous Mater., 2013; 180,187-195.
CrossRef - L. Hansheng, H. Shichao, M. Ke, W. Qin, J. Qingze, S. Kening. Appl. Catal. A: Gen., 2013; 450, 152-159.
CrossRef - Y. Sang, H. Liu, S. He, H. Li, Q. Jiao, Q. Wu, K. Sun. J Energy Chem., 2013; 22,769-777.
CrossRef - J. Qi, T. Zhao, F. Li, G. Sun, X. Xu, C. Miao, H. Wang, X. Zhang. J. Porous Mater.,2010; 17,177-184.
CrossRef - D. Kubička, L. Kaluža. Appl. Catal. A: Gen., 2010; 372, 199-208.
CrossRef - D. Han, N. Sun, J. Liu, C. Li, H. Shan, C. Yang. J Energy Chem., 2014;23, 519-526.
CrossRef - D.P. Serrano, J. Aguado, J.M. Rodriguez, A. Peral. J. Anal. Appl. Pyrolysis., 2007; 79, 456-464.
CrossRef - P. Liu, Z. Zhang, M. Jia, X. Gao, J. Yu. Chin. J. Catal., 2015; 36, 806-812.
CrossRef - L. Zhao, J. Gao, C. Xu, B. Shen. Fuel Process.Technol.,2011; 92,414-420.
CrossRef - J.A. Botas, D.P. Serrano, A. García, R. Ramos. Appl. Catal. B: Environ.,2014; 145, 205-215.
CrossRef - J.A. Botas, D.P. Serrano, A. García, J.de. Vicente, R. Ramos. Catal.Today.,2012; 195, 59-70.
CrossRef - D. Han, N. Sun, J. Liu, C. Li, H. Shan, C. Yang. J. Eng.Chem., 2014; 23, 519-526.
CrossRef - S. Bendezú, R. Cid, J.L.G. Fierro, A. LópezAgudo. Appl. Catal. A: Gen., 2000; 197, 47-60.
CrossRef - A. Ishihara, T. Itoh, H. Nasu, T. Hashimoto, T. Doi. Fuel Process.Technol.,2013; 116, 222-227.
CrossRef - M.A. Ali, T. Tatsumi, T. Masuda. Appl. Catal. A: Gen., 2002; 233, 77-90.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









