Adsorption Behavior of Alkali Metal (Na+, Li+, and K+) from Bledug Kuwu Brine by Resin Adsorbent for Purification: pH and Flow Rate Parameter
Miftakhur Rohmah1* , Latifa Hanum Lalasari1
, Latifa Hanum Lalasari1 , Nadia Chrisayu Natasha1
, Nadia Chrisayu Natasha1 , Eko Sulistiyono1
, Eko Sulistiyono1 , Florentinus Firdiyono1
, Florentinus Firdiyono1 and Johny Wahyuadi Soedarsono2
and Johny Wahyuadi Soedarsono2
1Research Center for Metallurgy and Materials, Indonesian Institute of Science, Building 470 PUSPIPTEK, South Tangerang, Indonesia
2Department of Metallurgy and Materials Engineering, Universitas Indonesia, Depok, Indonesia
Corresponding Author E-mail: miftakhur.rohmah@lipi.go.id
DOI : http://dx.doi.org/10.13005/ojc/360209
Article Received on : 16 Jan 2020
Article Accepted on : 17 Mar 2020
Article Published : 13 Apr 2020
In this study, the adsorption behavior of Na+, Li+, and K+ from an oxalate solution using polymer cation exchange, Lewatit S-108 resin type sodium has been investigated. The oxalate solution was prepared at three conditions (pH 4, 6, and 12) then eluted to 50 gr resin with flow rates of 25, 50, and 100 ml/hour. During the process, the alkali metals ions compete to adsorb onto resin. Adsorption behavior was directed by adsorption and kinetic tests with Langmuir and Freundlich isotherms. It was found that the resin Lewatit S-108 has a high adsorption efficiency and a great selectivity for alkali metal ion in the oxalate solution. The amount of Li+ adsorption was ± 0.0030 - 0.0032 mmol/g, K+ was 0.0027 - 0.0028 mmol/g, and Na+ was not adsorb. The faster the flow rate is, the higher the efficiency of the acquisition of Li and K gets. The higher the pH is, the maximum the amount and adsorption capacity of Li and K get.
KEYWORDS:Adsorption; Alkali Metal; Bledug Kuwu; Lithium; Lewatit Resin
Download this article as:| Copy the following to cite this article: Rohmah M, Lalasari L. H, Natasha N. C, Sulistiyono E, Firdiyono F, Soedarsono J. W. Adsorption Behavior of Alkali Metal (Na+, Li+, and K+) from Bledug Kuwu Brine by Resin Adsorbent for Purification: pH and Flow Rate Parameter. Orient J Chem 2020;36(2). |
| Copy the following to cite this URL: Rohmah M, Lalasari L. H, Natasha N. C, Sulistiyono E, Firdiyono F, Soedarsono J. W. Adsorption Behavior of Alkali Metal (Na+, Li+, and K+) from Bledug Kuwu Brine by Resin Adsorbent for Purification: pH and Flow Rate Parameter. Orient J Chem 2020;36(2). Available from: https://bit.ly/2K2Bcoq |
Introduction
Purification is an important step in the advanced technology of waste. Since numerous valuable minerals are dissolved in mud volcanoes, like Cu, Pb, Zn, Mn, Fe, Cd, As, Sb, Au, Ag, Hg, and high concentration of salt NaCl, KCl, etc., the purification of each mineral from hydrothermal mudflow has become an interest. In general, the concentration of those elements is quite low, except for sodium and potassium. Bledug Kuwu is one of most mud volcanoes that is interpreted as a geothermal activity. Other active mud volcanoes in Indonesia are located in Sangiran, Mount Anyar, Lapindo Sidoarjo, and they were formed by rocks incorporated with the seawater intrusion [1] [2]. Miftakhur et al (2018) reported that the dry of Bledug Kuwu mud generally consists of 3.28% Na, 0.21% K, 0.004% Li, 1.06% Ca, and other valuable elements which are evenly distributed in Li-Montromorolonit, Quartz, Potassium Borodosilicate, and Feldspar [3].
Almost 80% of the worldwide brine deposit is concentrated in Chile (1500 ppm Li), Bolivia (970 ppm Li), and Argentina (600 ppm Li) [4]. However, brine deposit in Indonesia is found to be associated with mud volcanoes that accumulate to economic concentrations as specialized clays, a silicate mineral form. These elements particularly Li, Na, and K have incredible potential to be further processed, and thereby could increase the sale value of Bledug Kuwu. Not only being used as kitchen salt (NaCl), the release of salt-waters from the lava, mud, and fluid mud by mud volcanoes is utilized as a raw material of chemical and metallurgy industries for further processing of Li and K content.
Although alkali metals are not particularly rare metals, they can be used in general application. Alkali metals are essential for human life because it has great characteristics, i.e., low density, low melting point, and is rapidly reactive with oxygen and water vapors. These properties are due largely to the presence of single valence electrons on each shell of the atom. Although most natural alkali is never found as free metals, lithium (Li) has a beneficial effect at low concentration as lithium carbonate, lithium hydroxide, and lithium sulfate. Moreover, sodium (Na) is applied in the production of titanium metals and a small nuclear reactor, while potassium (K) is applied for heat exchange alloys. Li, Na, and K ions are subjected to metal anodes for rechargeable batteries with high capacities [5] [6]. Due to the important commercial aspect, alkali metals in all resources need to go through selective recovery or purification. In addition, the low levels of lithium and potassium in mud or soil makes it difficult to extract them profitably.
Since the brine of Bledug Kuwu is open to the environment, the concentration process with solar evaporation takes 18 to 24 months. Because of extremely low Li and K content in Bledug Kuwu compared to other salt-lakes in the world, adsorption is one of the effective techniques to recover or remove alkali metal ions from aqueous solution, economically. Adsorption technology has several advantages compared to other recovery methods from brine sources, such as selectively adsorbs certain ions according to ion radius, and is potentially developed on a large scale [4]. The research for lithium selective adsorbent has strengthened over the last several years. There are several adsorbents and ion exchanges for selective recovery alkali metals (Li, K, and Na) from brine solutions, such as: the spinel type manganese oxide, polymeric membrane containing an inorganic ion exchange, hydrated alumina, anion exchange resin containing active group of Al(OH)3, and resin [4] [7] [8]. Earlier investigations reported that cross-linked polymer matrix such as activated carbon, Amberlite IR 120 exchange resin, Mesostructured aluminosilicate MCM 41, and molecular sieve 13X were achieved at approximately 25 ppm Li with sorption/desorption methods [9]. Lewatit resin is proved to have superior loading capacity with mono-disperse. Nevertheless, the quality of the sorbent materials depends on sorption capacity, stability, and selectivity to other competitive bulk ions. On the other hand, it is well known that the effectiveness of sorption varies as it depends on the pH, the affinity for the resin phase, and the temperature [10]. The affinity for Lewatit SP is as follows [10]:
Ba2+ > Pb2+ > Sr2+ > Ca2+ > Ni2+ > Cd2+ > Cu2+ > Co2+ > Zn2+ > Fe2+ > Mg2+ > K+ > NH4+ > Na+ > H+ (1)
In this experiment, we did some improvisations in applying a commercial resin in Indonesia, Lewatit S-110 resin, for adsorption of Li (I), K (I), and Na (I) by using HCl solution and by modifying the procedure, selectively. The research was conducted using modified Bledug Kuwu brine with an oxalate solution. To obtain a higher separation and concentration of alkali metals, different pH and flow rates of solution were used.
Materials and Method
Preparation of Bledug Kuwu Brine
Alkali metal solutions (Li, K, and Na) can be leached out of Mud Volcano of Bledug Kuwu by a water leaching method, as done by Miftakhur et.al [3] to extract alkali metals from Bledug Kuwu. The oxalate solution was made by adding 23.5 milligrams per ml brine of Bledug Kuwu. The chemical composition of solution is presented in Table 1.
Table 1: Chemical Composition of Modified Brine Bledug Kuwu.
|
Calcium |
Potassium |
Lithium |
Magnesium |
Sodium |
|
10.95 ppm |
115.30 ppm |
22.30 ppm |
0.14 ppm |
2016.26 ppm |
Preparation of Resin Lewatit S-108
In this experiment, Lewatit S-108 resin, Gel type resin, is selected for the adsorption study, which has a hetero-disperse size of 300 – 800 μm for sodium form (Figure 1). As can be seen in Figure 2, Lewatit resin is classified as sulfonic acid type which has a cross-linked polystyrene matrix with divinylbenzene, while it is indicated by the sulfonate function (S=O stretching) at wave numbers 1159.13 cm-1 and 1123.15 cm-1. Moreover, the other wave numbers are known as asymmetric vibration of sulfonic group SO3–. Gel type resin is able to absorb organic substances from an oxalate solution depending on the degree of porosity and the functional groups, which is presented in Figure 2. The resin with higher cross-linking is low resistant to degradation by oxidizing agents such as chlorine. Resin can operate from pH 0 to 14.
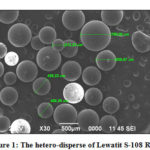 |
Figure 1: The hetero-disperse of Lewatit S-108 Resin |
 |
Figure 2: Functional group of Lewatit S-108 Resin by FTIR analysis |
Because the resin used in this research was Na+ type, resin activating using HCl was expected to replace Na+ in resin with H+ in HCl solution. Resin activating was carried out by immersing 50 grams resin into 50 ml 1M HCl solution for 24 hours, then, it was rinsed with distilled water until the TDS approached 2μS/cm. The bond between sodium and sulfonate resin (Nares) was replaced by an acid (H+) from HCl according to Eq.1.
Na+-R-SO3 + HCl →H+-R-SO3 + NaCl (Equation 1)
Adsorption Experiment
Concentration of lithium, sodium, and potassium in solution were determined by the Inductively Coupled Plasma – Optical Emission Spectroscopy (ICP-OES) which has a detection limit in ppb levels. Prior to the sorption experiment, the solution had to be free from precipitation in the form of oxalate compounds at selected pH. The adsorption experiment was completed by a column separation, with 2 cm diameter column, 20 cm bed height, which is displayed in Figure 3. The effect of pH solution on alkali adsorption in resin was determined by adjusting at 4, 6, and 12.
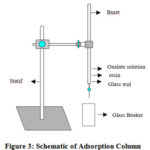 |
Figure 3: Schematic of Adsorption Column |
In the batch experiment, each cycle of the adsorption process consisted of three stages: (1) Loading the slurry resin with 25 ml HCl 0.1 M for activating the functional group into column (2) Entering the modified solution (Bledug Kuwu Brine) with various pH and controlling the flow rate at 25, 50, and 100 ml/hour, called as eluent (3) Attaching elution and desorption of Na, Li, K ions to the adsorbed resin, called as effluent (4) Regenerating resin with 2N HCl solution.
The effluent contained in the glass beaker was tested by ICP to determine the different concentration and then calculated for final concentration, distribution coefficient, and resin performance. The adsorption number of ions onto resin was calculated with Eq.2, while yield adsorption was calculated using Eq.3.


In the equation, q is the adsorption number of ions onto resin (mmol/gr). Ci and Cf are initial and final concentration at equilibrium condition (mg/L mM), respectively; m is the mass of resin dry weight (gr), and V is the volume of brine which is loaded into the column (L). In order to have a total comparison for the adsorption, the ability of adsorbent to selectively adsorb certain ions by comparing the competitor ions is called a separation coefficient, which is calculated using Eq.4.

In addition, the kinetic studies were relatively well modeled by Langmuir Equation (monolayer adsorption) and Freundlich equation (multilayer adsorption). The Langmuir isotherm model is given in Eq.5, and the Freundlich isotherm models are expressed in Eq.6 and Eq.7.

Ce is the concentration of alkali metals at an equilibrium state (mg/l). Qe is the amount of alkali metals that adsorbed per gram of resin at an equilibrium state (mg/g). B is adsorption constant to theory / adsorption energy (L/mg). Qm is the maximum adsorption capacity. Qm and b can be depicted as Langmuir isotherm constant that expects an amount of adsorption at monolayer formation (mg/g). Moreover, Kf is Freundlich isotherm constant (dm3/g) and n is exponent from Freundlich models. It can be determined from the slope and intercept between log Qe and log Ce line.
Result
Sorption Behavior
The column experiment performed the adsorption of alkali metals from the modification of the Bledug Kuwu brine with oxalate on Lewatit resin. After the adsorption test, brine of Bledug Kuwu has different chemical composition, as shown in Table 2. Generally, significant differences were seen at Li and K; they reduced drastically. Nevertheless, there were no significant changes on Na level. It indicates that the adsorption process only occurs on Li and K onto Lewatit resin on the oxalate solution.
Table 2: Characterization of Effluent after Adsorption Test
|
Sample Code |
Parameter |
Concentration (ppm) |
Final pH |
Amount of Adsorption (mmol/gram) |
|||||
|
Flow rate |
pH |
Li |
K |
Na |
Li |
K |
Na |
||
|
Feed |
– |
2 |
22.30 |
115.31 |
2016.26 |
1 |
– |
– |
– |
|
R1 |
25 ml/hour |
4 |
0.02 |
4.83 |
2089.14 |
1 |
0.00321 |
0.00283 |
-0.0032 |
|
R2 |
6 |
0.2 |
4.4 |
2935.4 |
1 |
0.00318 |
0.00284 |
-0.04 |
|
|
R3 |
12 |
0.03 |
4.57 |
2128.37 |
1 |
0.00321 |
0.00283 |
-0.0049 |
|
|
R4 |
50 ml/hour |
4 |
1.76 |
7.89 |
2084.73 |
1 |
0.00296 |
0.00275 |
-0.003 |
|
R5 |
6 |
1.26 |
6.18 |
2053.16 |
1 |
0.00303 |
0.00279 |
-0.0016 |
|
|
R6 |
12 |
0.36 |
5.41 |
2055.42 |
1 |
0.00316 |
0.00281 |
-0.0017 |
|
|
R7 |
100 ml/hour |
4 |
0.11 |
5.97 |
2070.27 |
1 |
0.0032 |
0.0028 |
-0.0024 |
|
R8 |
6 |
0.05 |
5.85 |
2103.30 |
1 |
0.00321 |
0.0028 |
-0.0038 |
|
|
R9 |
12 |
0.04 |
4.41 |
2133.18 |
1 |
0.00321 |
0.00284 |
-0.0051 |
|
In the present study, the order of alkali metals adsorption in the oxalate solution is Li > K > Na onto Lewatit resin. This phenomenon is caused by the size of radius Li (r=90) which tends to be smaller than K (r=152) and Na (r=116), respectively. As can be seen in Table 2, the amount of Li adsorption is ± 0.003 – 0.0032 mmol/gr, adsorption of K is 0.00274 – 0.00284 mmol/gr, and Na adsorption is negative. It indicates that Na cannot be adsorbed, because the Lewatit resin used is Na type. Furthermore, there is Na left on a solid surface of the resin during the activation process with HCl. The adsorption model needs to be studied further.
In addition, Neuman (2010) showed that H+ only attached to the resin surface without a cation exchange mechanism into Lewatit resin [11]. The H+ was predicted to be trapped in resin pores like in Equation 8, as the lithium ion has a similar radius as hydrogen that lithium may be adsorbed easier. On the other hand, K+ is partially adsorbed into resin (Equation 9). The results of this study are similar to Guo et al (2013) who stated that Lewatit resin can act as an absorber and an exchanger because of the presence of dual adsorption mechanism. Li+ is more likely to form ion complexes (oxalate) in the outer sphere while K+ form complexes in the inner sphere during the adsorption process so that Li + ions are adsorbed with the original hydration skin [12]. Li ion tends to be physically adsorbed without ion exchange mechanism, while K ions are electrostatically adsorbed (cation exchange).
Na-res(s) + Li+(aq) à Li-res(s) (on resin surfaces) + H+ + Na-res (Equation 8)
Na-res(s) + H+ + K+(aq) à K-res(s) + H+ + Na+ (Equation 9)
In order to have a comparison for the adsorption, a separation factor was calculated according to the Eq. 2. The separation factor of other elements which is compared to lithium is followed as: β Ka/Li > β Na/Li under all condition, which is presented in Table 3. This confirms that Lewatit resin is more favorable selectivity for potassium (K+) than sodium (Na+).
Table 3: Coefficient Separator for Li
|
Sample Code |
Parameter |
Coefficient Separator (ml/gr) |
||
|
Flow rate |
pH |
K/Li |
Na/Li |
|
|
R1 |
25 ml/hour |
4 |
0.021 |
0.00031 |
|
R2 |
6 |
0.228 |
0.00283 |
|
|
R3 |
12 |
0.033 |
0.00071 |
|
|
R4 |
50 ml/hour |
4 |
1.006 |
0.00281 |
|
R5 |
6 |
1.058 |
0.00108 |
|
|
R6 |
12 |
0.333 |
0.00031 |
|
|
R7 |
100 ml/hour |
4 |
0.006 |
0.00013 |
|
R8 |
6 |
0.042 |
0.00093 |
|
|
R9 |
12 |
0.045 |
0.00095 |
|
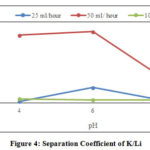 |
Figure 4: Separation Coefficient of K/Li |
In general, pH, flow rate, and combination of both affects the mount of adsorption. As can be seen in Figure 4, the highest separation coefficient (β Ka/Li) is obtained while the flow rate is 50 ml/hour and pH 6. The pH affects the active site load from the surface of the adsorbent and affects the solubility of metal ions in solution. At acidic pH, Li and K tend to form certain compounds, so the separation coefficient tends to be lower [8]. The number of active loads is consistent with the ionic bonding. The high value of coefficient separation is caused by the degree of crosslinking of the resin and does not depend on the resin shape [11].
Desorption Behavior
Desorption is the removal process of ions adsorbed onto Lewatit resin using a regenerated solution. Li+ and K+ were replaced with H+ from HCl, and they were removed on strong adsorption resin. The ICP test results of regenerated solution are shown in Table 4. Overall, the desorption process can remove K+ and Li+, partially. After the regeneration, the recovery of Li+ ranges from 4-6 ppm, while K+ ranges from 50 to 85 ppm.
Table 4: Chemical Composition of Eluent after Desorption
|
Sample Code |
Composition (ppm) |
||
|
Li |
K |
Na |
|
|
Feed |
22.30 |
115,31 |
2016,26 |
|
R1 |
6,10 |
80,86 |
1107,48 |
|
R2 |
5,56 |
82,85 |
1191,33 |
|
R3 |
5,66 |
61,62 |
863,06 |
|
R4 |
5,181 |
65,11 |
2064,72 |
|
R5 |
6,584 |
75,499 |
2034,26 |
|
R6 |
5,978 |
84,737 |
1987,81 |
|
R7 |
5,70 |
54,74 |
869,34 |
|
R8 |
4,57 |
46,54 |
766,81 |
|
R9 |
6,56 |
63,44 |
986,54 |
The yield adsorption of lithium and potassium in all conditions are presented in Figure 5. Generally, the yield adsorption of lithium is similar to potassium, which is below 50%. The optimum condition of lithium adsorption is obtained by base and acidic environment and fast flow rate. On the other hand, the optimum condition for potassium adsorption is in the moderate flow rate. Lithium tends to form a compound, lithium stable cation at acidic and base pH, so these compounds are more easily adsorbed into resin. It generally follows the Hofmeister series and the surface tension [13]. On other hand, Karamui et al (2009) stated that by using 1 M HCl obtained, the yields of Li, K, and Na separation were 34.8%, 2.38%, and 18.4% at 20 gr AG 50W-X8 resin.
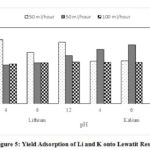 |
Figure 5: Yield Adsorption of Li and K onto Lewatit Resin |
Indirectly, K+ bound to the resin, roughly are 5.467 mg at pH 4; 5.473 mg at pH 6; and 5.545 mg at pH 12. While Li+ are 1.1095 mg at pH 4; 1.1125 mg at pH 6; and 1.113 mg at pH 12. As can be seen in Figure 5, stability of ions absorbed shows that the adsorption of K and Li ions onto Lewatit resin is not significant, which is affected by pH Lewatit resin can adsorb Li and K depending on the size of atomic radius. Furthermore, the variety in flow rate was predicted to have a dominant influence on the adsorption process.
Discussion
Isotherm Model
Langmuir Models
Langmuir adsorption of kinetic model depends on the following assumptions: adsorption rate will rely upon the size and molecular structure, solvent properties and porosities, and active sites on the homogeneous surface of adsorbent during a monolayer formation [13].
As indicated, the monolayer theoretical depends on the Langmuir Isotherm Models, and the maximum of adsorption capacity with monolayer system is 0.4 – 0.44 mg/g on Li+ and 2 – 2.12 mg/g on K+, empirically. In an equilibrium adsorption, the binding process of each ion increases with the charge increases, but it decreases with the radius of hydrated ionic. The hydrated radius of potassium is smaller than lithium and has the possibility to make the bonding more efficient. Moreover, the maximum of adsorption capacity increases with increasing pH (near base solution). This is demonstrated by the adsorption yield which expresses that the presence of more OH– will adsorb more positively charged ions.
The kinetic models according to the Langmuir equation is depicted in Figure 6. Models of Langmuir isotherm adsorption can be applied to the adsorption process onto Lewatit resin. This is indicated by the value of R2 is 1 in each graph of lithium and potassium (Fig 6a and 6b).
 |
Figure 6: Langmuir Models for (A) Lithium (B) Potassium of Adsorption onto Lewatit Resin |
Freundlich Models
Based on Freundlich isotherm models, the heterogeneous adsorption process has two phases, namely: (A) the transfer of ions from the solution to adsorbent surface, and (B) Adsorption mechanism presents on adsorbent surface. The kinetic models according to the Freundlich equation are exhibited in Figure 7.
 |
Figure 7: Freundlich Models for Alkali Metals Adsorption onto Lewatit resin |
Conclusion
The adsorption order in the form of oxalate substance is as follows: Li > K > Na. The Freundlich equation was found to describe the mechanism of alkali metals adsorption onto Lewatit resin more accurately than the more Langmuir equation.
Acknowledgments
The authors are grateful for the financial support through the INSINAS fund (Director General of Research and Development Strengthening of Letter Decree 4/E/KPT/2019 on 1 February 2019 and contractual agreement Number of 088/P/RPL-LIPI/INSINAS-1/II/2019) from the Ministry of Research, Technology, and Higher Education.
Conflict of Interest
All authors have read and approved the manuscripts and take full responsibility for its content. The authors have no conflict of interest in regard to this research or its funding.
References
- Siregar,S.; and Siregar, N. I. POSITRON. 2016, 1, 40-42. (In Indonesia)
- Hidayati, D.; Othman M, I. B.S, S.; Sulaiman, N, Sains Malaysiana, 2018,47,1665-1674.
- Rohmah, M.; Lalasari, L. H.; Wahyuadi, J.; Natasha, N. C, IEEE International Conference on Innovative Research and Development (ICIRD). 2018
- Choubey, P. K.; Kim, M.-s.; Srivastava, R. R.; Lee, J.-c; Lee, J.-Y, Minerals Engineering. 2016, 89, 119-137.
- Wang, H.; Yu, D.; Kuang, C.; Cheng, L.; Li, W.; Feng, X.; Zhang, Z.; Zhang, X; Zhang, Y, Chem. 2019. 5. 313-338.
- Mc Quarrie, R. a. G, “Interchapter D: The Alkali Metals,” in General Chemistry, Fourth Edition, USA, University Science Books, 2011, Chapter D.
- Meshram, P.; Pandey, B.; Mankhand T., Hydrometallurgy, 2014, 150, 192-208.
- Karami, H.; Sadeghi, M.; Nemati, S.; Zeinali, B., Analytical Chemistry an Indian Journal, 2009. 8. 109-113.
- Lemaire, J.; Svecova, L.; Lagallarde, F.; Laucournet, R.; Thivel, P.-X., Hydrometallurgy, 2014. 143. 1-11.
- Hubicki, Z.; Kolodynska,D., Environmental Science. 2012. 193-240.
- Neuman, S.; Fries, G.; Bjorn,D., LENNTECH LANXESS Engineering Chemistry. 2010. 1-22.
- Guo, T.; Wang, S.; Ye, X.; Liu, H.; Guo, X.; Li, Q. Desalination and Water Treatment, 2013. 51. 3954-3959.
- Perrine, K. A.; Parry, K. M.; Stern, A. C.; Van Spyk, M. H.; Makowski, M. J.; Freites, J. A.; Winter, B.; Tobias D. J.; Hemminger, J. C., Proceedings of the National Academy of Sciences of the United States of America, 2017. 114. 51. 13363-13368.

This work is licensed under a Creative Commons Attribution 4.0 International License.









