Unexpected synthesis of three components from the reaction of 4-chloro-3-formylcoumarin with hydroxylamine hydrochloride
Ahmed Elkhateeb1,2, Ashwag S. Alshehri1, Gilbert Kirsch3 and Sh. H. Abdel-Hafez1,4
1Department of Chemistry, Faculty of Science, Taif University, Taif 21974, Saudi Arabia
2Phytochemistry and plant systematic department, National Research Centre, Dokki, 12622 Giza, Egypt
3UMR 7565 SRSMC, Boulevard Arago, 57070 Metz, France
4Department of Chemistry, Faculty of Science, Assiut University, Assiut 71516, Egypt
Corresponding Author Email: shams_abdelhafez@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320142
Article Received on :
Article Accepted on :
Article Published : 25 Jan 2016
A mild and simple oximation reaction of 4-chloro-3-formylcoumarin with hydroxylamine hydrochloride in basic medium gave unexpected three components via oxime. Those compounds were separated in good yields by preparative TLC and by chemical means using different reaction conditions. IR, 1H NMR, 13C NMR and mass spectral data confirmed the structure of the separated compounds.
KEYWORDS:4H-coumarin[3,4-d]isoxazol-4-one; ethyl-5-(2-hydroxyphenyl)-isoxazole-4-formate; methyl-5-(2-hydroxyphenyl)-isoxazole-4-formate; 4-chloro-3-cyano-coumarin
Download this article as:| Copy the following to cite this article: Elkhateeb A, Alshehri A. S, Kirsch G, Abdel-Hafez S. H. Unexpected synthesis of three components from the reaction of 4-chloro-3-formylcoumarin with hydroxylamine hydrochloride. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Elkhateeb A, Alshehri A. S, Kirsch G, Abdel-Hafez S. H. Unexpected synthesis of three components from the reaction of 4-chloro-3-formylcoumarin with hydroxylamine hydrochloride. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13636 |
Introduction
Derivatives of 4H-1-benzopyran-4-one, also known as 4H-chromen-4-ones or chromones, are an important scaffold in heterocyclic chemistry and represent useful synthetic building blocks in organic and medicinal chemistry [1]. Coumarins or Chromones are natural products possessing a wide range of valuable physiological activities [2]. From literature survey the syntheses of 2-aminochromone-3-carboxamide, 3-amino-4H-chromeno[3,4-d]isoxazol-4-one, and 3-(diaminomethylene) chroman-2,4-dione were developed [3] from the reactions of 3-substituted chromones (3-formylchromone, 3-formylchromone-3-oxime, and 3-cyanochromone) with hydroxylamine in alkaline medium.3-Formylchromones when treated with hydroxylamine formed via 3-formylchromone-oximes 3-cyanochromones[4, 5]. The ability of 3-
cyanochromones to interact with water followed by pyrone ring opening and cyclization at the CN group formed 2-amino-3-formylchromone [5, 6].
In this study we have investigated the reaction of 4-chloro-3-formylcoumarin with hydroxylamine hydrochloride at different conditions to see if the reaction will have the same behavior as previously described in literature [3].
Results and Discussion
The literature survey revealed some debate about the assignment of the structure of the reaction products of coumarin derivatives with hydroxylamine.
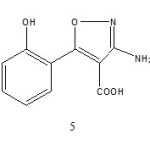
Among these investigations, Sosnovskikh et al. in 2008 [3] studied the reactions of chromones 1-3 with hydroxylamine hydrochloride in strongly basic medium to give 3-amino-4H-chromeno[3,4-d]isoxazol-4-one 4 which was converted into acid 5 when heated in 10 % boiling sodium hydroxide.
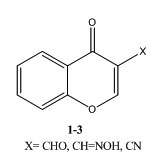
Further recyclization and reduction of compound 4 led to the formation of 3-(diaminomethylene) chroman-2,4-dione 6.
 |
Scheme Click here to View scheme |
 |
Scheme 1 Click here to View scheme |
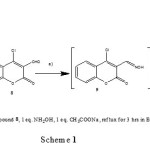
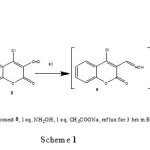
Therefore, we studied the reaction of 4-chloro-3-formylcoumarin 8 instead of compounds 1-3 with hydroxylamine under different conditions. We identified three unexpected compounds; 4-chloro-3-cyano-coumarin (10); 4H-chromeno[3,4-d]isoxazol-4-one (11); Ethyl-5-(2-hydroxyphenyl)-isoxazole-4-formate (12a); (or methyl-5-(2-hydroxyphenyl)-isoxazole-4-formate (12b) (depending on the solvent) formed through oxime (9) (Scheme 1).
 |
Scheme 1 A Click here to View scheme |
These 3 compounds were prepared as follow: 1equivalent of starting compound 8 with 1eq. of hydroxylamine hydrochloride in the presence of 1eq. of sodium acetate as a basic medium were refluxed for 4 hrs . Formation of compounds 10-12 was monitored by TLC and GC/MS. When the reaction was carried out by using 3eq hydroxylamine/ 3eq sodium acetate in ethanol and was refluxed for 10 hrs. pure compound 11 was isolated and the structure confirmed on the basis of IR, 1HNMR, 13CNMR and mass spectra. A characteristic singlet peak at δ 10.21 due to CH-isoxazole moiety was present in 1HNMR. Treatment of chromones 8 (1eq) with 3eq of hydroxylamine and 3eq of sodium acetate under reflux 24hrs. gave pure 12 a in ethanol or 12 b in methanol (Scheme 2).
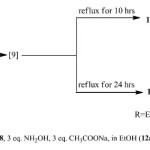 |
Scheme 2 Click here to View scheme |
Structures of 12a and 12b were confirmed on the basis of spectral analysis. Compound 10 was obtained directly when the reaction was carried out under stirring 5 minutes by using 3eq hydroxylamine/3eq sodium acetate in ethanol as a solvent (Scheme 3).
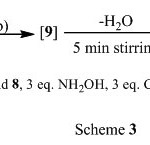 |
Scheme 3 Click here to View scheme 3 |
It was found that the spectral data of 10 were in agreement with literature results [7] (m.p.199-200oC). These compounds 10-12 were separated and purified by chromatography using hexane/ethyl acetate (8:2) as eluent.
Concerning the mechanism 10 was formed by elimination of water from oxime (9); 11 was formed by elimination of HCl to ring closure. Finally, the ethylester isoxazole 12a was obtained via ring opening of coumarin (lactone) by the action of ethanol.
In order to study the effect of solvent, we have repeated the reaction in methanol instead of ethanol to give methylester isoxazole 12b (Scheme 2).
A possible route for the multi-step mechanism is outlined in (Scheme 4).
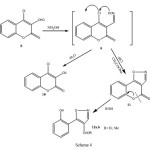 |
Scheme 4 Click here to View scheme |
We suggest that the hydroxylamine in basic medium converts 8 into the key intermediate compound 9 which then undergoes further recyclization by elimination of HCl to afford coumarino[3,4-d]isoxazole 11. Ethanol or methanol makes the ring opening of the lactone part to form compounds 12a,b.
In conclusion, the starting compound 4-chloro-3-formyl coumarin 8 showed different reactivity depending on the reaction conditions. Its reaction with hydroxylamine gives a variety of products. The obtained products were 4H-coumarino[3,4-d]isoxazol-4-one; ethyl-5-(2-hydroxyphenyl)-isoxazole-4-formate or methyl-5-(2-hydroxyphenyl)-isoxazole-4-formate) and 4-chloro-3-cyano-coumarin.
Experimental Section
General Procedures
Melting points were determined by using the Kofler melting point apparatus, and were uncorrected. IR (KBr, cm-1) spectra were recorded on a Pye-Unicam SP3–100 instrument at Taif University. 1H NMR spectra were obtained on a Varian (400 MHz) EM 390 USA instrument at King Abdel-Aziz University. 13 C NMR spectra were recorded on a JNM-LA spectrometer (100 MHz) at King Abdel-Aziz University, Saudi Arabia. For both 1H and 13C –NMR, DMSO d6 was used. Spectra were internally referenced to TMS. Peaks are reported in ppm downfield of TMS. Multiplicities are reported as singlet (s), doublet (d), triplet (t), quartet (q).
Mass spectra were recorded on ISQ Thermo Scientific GC-MS. GC column TG-SQC, Trace GC ultra at TaifUniversity KSA.Purity of the compounds was checked by thin layer chromatography (TLC) using silica gel plates.Column chromatography was carried out on 0.04−0.063 mm(Merck) silica gel, thin layer chromatography was carried out on aluminum backed silica plates by Merck and plates were revealed using a UV 254 light.
Materials
The 4-hydroxycoumarin 7 was a gift from the Lab of Prof. Gilbert Kirsch, Laboratoired’Inge´nierie Mole´culaire et Biochimie Pharmacologique, Institut Jean Barriol, FR Metz, France..
4-Chloro-3-coumarincarbaldehyde (8)
Compound 8 was prepared as previously describe by sabitaeet.al with a little bit modification [8].
To a stirred mixture of 4-hydroxycoumarin 7 (9.72 g, 0.06 mol) in anhydrous DMF (46.2 mL, 0.6 mol) were added drop wise POCl3 (27.6 g, 0.18 mol) at –10° to –5 °C. The reaction mixture was then stirred for 1 hr at room temperature after that heated and stirred for 5 hrs. at 80 °C. The reaction mixture was poured onto crushed ice (300 g) under vigorous stirring. The reaction mixture was kept overnight at 0°C. The pale yellow solid was collected by filtration and recrystallized from acetone to give 10.5 g (84%) of 8 ; m.p. 133-135°C(lit. 130 °C) [9]
1H-NMR δ 10.39 (1H, s, CH=O), 8.16–7.28 (4H, m, Ar-H); IR ν 1720 (C=O-pyrone), 1663 (CHO) cm–1. EI-MS m/z: 208 (M+, 11), 182 (31), 180 (100), 154 (31), 152 (91), 124 (20), 101 (11), 89 (80), 63 (37), 62 (31), 61 (14); EI-HRMS: m/z 207.9909 (calculated for C10H5ClO3 207.9927)
3-Cyano-4-chlorocoumarin (10), 4H-chromeno [3,4-d]isoxazol-4-one (11), ethyl 5-(2-hydroxyphenyl)isoxazole-4-carboxylate (12a) and Methyl-5-(2-hydroxyphenyl)-isoxazole-4-formate (12b)
General Procedure
To a mixture of NH2OH.HCl (1 eq.) and anhydrous sodium acetate (1 eq.)in ethanol, or methanol a solution of compound 8 (1 eq.) in ethanol was added. The reaction mixture was refluxed for 3hrs. After the reaction completed, the reaction mixture was poured onto crushed ice (300 g) under vigorous stirring. The pale yellow solid was collected by filtration and further purified by silica gel column chromatography using a mixture of hexane/ethyl acetate (8:2) as eluent to afford the desired three compounds10, 11 and 12a,b.
Notes: Compound 10 was prepared by using 3eq of NH2OH.HCl/ 3eq anhydrous sodium acetate and 1eq of compound 8 in ethanol. The reaction mixture was stirred for 5 minute to give compound 10 as yellow crystal m.p. 198-200°C (Lit, 199-200 °C)[9]
4H-Chromeno [3,4-d]isoxazol-4-one (11)
m.p. 222-224 °C; 1H-NMR δ 10.21 (1H, s, CH-isoxazole), 8.03–7.41 (4H, m, Ar-H); 13C-NMR δ 165.50, 155.04, 154.90, 152.80, 133.36, 125.29, 124.07, 117.67, 110.24, 107.83. IR ν 1761 (C=O-pyrone), 1608 (C=N) cm–1. EI-MS m/z: 187 (M+, 100), 159 (33), 131 (11), 119(4), 103 (54), 76 (26). EI-HRMS: m/z 187.0268 (calculated for C10H5NO3: 187.0269)
Ethyl-5-(2-hydroxyphenyl)-isoxazole-4-formate (12a)
m.p. 218-220°C;1H-NMR (CDCl3) δ 9.02 (1H, s, CH-isoxazole), 8.12–7.01 (4H, m, Ar-H), 4.37 (2H, q, J= 6.80 Hz, CH2); 1.36 (3H,t, J= 6.80 Hz, CH3).13C-NMR δ 175.80, 163.49, 160.60, 155.68, 134.01, 125.66, 122.80, 118.68, 116.20.93.54, 60.29, 13.72.IR ν 1669 (C=O…H-bond), 1601 (C=N) cm–1. EI-MS m/z233 (M+, 33), 187 (100), 159 (41), 131 (12), 119 (4), 103 (35), 76 (8); EI-HRMS:m/z 233.0684 (calculated for C12H11NO4: 233.0688)
Methyl 5-(2-hydroxyphenyl)isoxazole-4-carboxylate (12b)
m.p. 210-212°C;1H-NMR (CDCl3) δ 9.02 (1H, s, CH-isoxazole), 8.12–7.01 (4H, m, Ar-H), 3.91 (3H, s, OCH3). IR ν 1670 (C=O…H-bond), 1600 (C=N) cm–1. EI-MS m/z219 (M+, 49), 187 (100), 159 (34), 131 (15), 119 (4), 103 (99), 76 (40). EI-HRMS: m/z 219.0492 (calculated for C11H9NO4: 219.0532)
References
- Nohara, A.; Kuriki, H.; Saijo, T.; Sugihara, H.; Kanno, M.; Sanno, Y. J. Med. Chem. 1977, 20, 141–145; (b) Nohara, A.; Umetani, T.; Ukawa, K.; Sanno, Y. Chem. Pharm. Bull. 1974, 22, 2959–2965; (c) Ukawa, K.; Ishiguro, T.; Wada, Y.; Nohara, A. Heterocycl., 1986, 24, 1931–1941; (d) Hsung, R. P. J. Org. Chem. 1997, 62, 7904–7905; (e) Hsung, R. P.; Zificsak, C. A.; Wei, L.-L.; Zehnder, L. R.; Park, F.;Kim, M.; Tran, T.-T. T. J. Org. Chem. 1999, 64, 8736–8740; (f) Sosnovskikh, V. Ya.; Irgashev, R. A.; Levchenko, A. A. Tetrahedron, 2008, 64, 6607–6614.
- Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893–930.
CrossRef - Vyacheslav Ya. Sosnovskikh , Vladimir S. Moshkin, Mikhail I. Kodess Tetrahedron Lett., 2008, 49, 6856–6859
- (a) Nohara, A. Tetrahedron Lett. 1974, 1187–1190;
CrossRef
(b) Klutchko, S.; Cohen, M. P.;Shavel, J.; von Strandtmann, M. J. Heterocycl. Chem. 1974, 11, 183–188.
CrossRef - (a) Petersen, U.; Heitzer, H. Liebigs Ann. Chem. 1976, 1659–1662; (b) Hagen, H.; Nilz, G.; Walter, H.; Landes, A.; Freund, W. DE Patent 4039281, 1992; Chem. Abstr.1992, 117, 111473k. Nohara, A.; Ishiguro, T.; Ukawa, K.; Sugihara, H.; Maki, Y.; Sanno, Y. J. Med. Chem. 1985, 28, 559–568.
CrossRef - Stojadin V. Dekić1, Vidoslav S. Dekić1, Branimirka Vučić2, Biljana R. Dekić1, Milan S. Dekić3. Physics, Chemistry and Technology, 2007, 5 (1) 85 – 88; Sabatie A., Vegh D., Floch L,, Arkivoc ,2001 (vi) 122-128
- Andrieux J., Battioni, J.P., Giraud M., Molho D., Bull.Soc. Chem. Fr. 1973, 6, 2093

This work is licensed under a Creative Commons Attribution 4.0 International License.









