Thermodynamic study of Cr+3 ions removal by "MnO2/MWCNT" nanocomposite
Shima Mohammadkhani1,*, M. R. Gholami2, 3 and M. Aghaie4
1Department, Science and Research Branch, Islamic Azad University, Tehran, Iran 2Chemistry Department, Science and Research Branch, Islamic Azad University, Tehran, Iran 3Faculty of Chemistry, Sharif University of Technology, Tehran, Iran 4Faculty of Chemistry, North Tehran Branch, Islamic Azad University, Tehran, Iran. Corresponding Author Email: shima_mohammadkhani@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320167
Article Received on :
Article Accepted on :
Article Published : 26 Jan 2016
In this research "MnO2/MWCNT" nanocomposite was prepared firstly and then it was used as an adsorbent for Cr+3 ions removal from aqueous solutions. Our results showed that the prepared nanocomposite from modified multi-wall carbon nanotube and MnO2 has a good capacity for Cr+3 removal from aqueous solution. Morphology and Crystallinity of the modified MWCNT before and after deposition on MnO2 were examined by SEM and XRD. In turn, the experimental results were examined according to the Langmuir, Freundlich and Temkin Isotherms and Freundlich isotherm represented our experimental results.
KEYWORDS:adsorption; adsorbent; isotherm; MnO2/MWCNT
Download this article as:| Copy the following to cite this article: Mohammadkhani S, Gholami M. R, Aghaie M. Thermodynamic study of Cr+3 ions removal by "MnO2/MWCNT" nanocomposite. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Mohammadkhani S, Gholami M. R, Aghaie M. Thermodynamic study of Cr+3 ions removal by "MnO2/MWCNT" nanocomposite. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13688 |
Introduction
Main reason causes environmental problems are toxic metal ions. Chromium is one of the most hazardous heavy metals [1]. It affects on human health, some problems that caused by chromium (III) are as fallow; lung cancer, kidney and liver damage, skin rashes and also it affects human physiology [2]. Although chromium (III) has use in so many industries like leather tanning, paints, glass manufacture and etc [3]. But the most significant point about chromium (III) is, it can be oxidized to Chromium (VI) that cause serious health risks [4,5]. Also admissible amount of chromium (III) in wastewater is 5mg/l [6]. So the elimination of chromium (III) from wastewater actuate tremendous attempt. There are prevalent methods for chromium (III) removal from the industrial wastewater consist of coagulation [7] and adsorption [8], but among these physicochemical [9] wastewater treatment; adsorption is a promising way for water reuse requirements. There are divers materials used as adsorbents but activated carbon is the most effective adsorbent for this application [10]. The amount of Chromium is very rarely in environmental water samples, so very sensitive techniques are used for the determination of chromium in water samples such as flame atomic absorption spectrometry (FAAS) [11], inductively coupled plasma-atomic emission spectrometry (ICP-AES) [12], graphite furnace atomic absorption spectrometry (GFAAS) [13] and inductively coupled plasma-mass spectrometry (ICP-MS) [14]. The purpose of this research is to extend advantages over other wastewater purification processes, and characterizing major factors.
Materials and Methods
Multi-wall carbon nanotube (MWCNT, Outer diameter: 8-15 nm, Length: 50 µm) were purchased from Neutrino, the initial solution and after adsorption were analyzed by Perkin Elmer Analyst 200, and pH meter (ABTGP353). A stock solution was prepared by dissolving 0.256 g CrCl3.6H2O in 50 cc distilled water. The pH values of the solution were adjusted to the desired values with either 0.1 mol/L HCl or 0.1 mol/L NaOH solutions. All chemicals were of analytical grade (AR) provided from Merck.
Preparation of MnO2/MWCNT nanocomposite
The preparation process for the MnO2/MWCNT nanocomposite was a co-precipitation method that before described by Zheng et al [15].
We synthesis MnO2/MWCNT nanocomposite. First of all we modify pure MWCNT by dispersing MWCNT in HNO3 65 wt% for 24 h under stirring then rinsed with deionized water and dried in the air at 100° C[16]. Afterwards, about 0.1 g of the treated MWCNT was dispersed into 160 ml of 0.1m KMnO4 solution under vigorous stirring at 40 °C for 2 h and 20 ml MnSO4 (0.5 M) solution was added dropwise on the above suspension with intensive stirring and then the reaction mixture was kept at 40 C° for 24 h. Then the suspension was filtered washed several times with deionized water and alcohol, and the precipitation were collected and dried in an oven under 100 C ° about 12 h.
Characterization of the treated MWCNT and MnO2/MWCNT nanocomposite
In order to determine crystallization and surface morphology, we used XRD diffraction and SEM techniques.
 |
Figure 1: XRD patterns of (a) activated MWCNT, (b) MnO2/MWCNT nanocomposites. Click here to View figure |
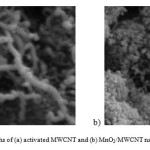 |
Figure 2: SEM photographs of (a) activated MWCNT and (b) MnO2/MWCNT nanocomposite. Click here to View figure |
It can be describe the XRD patterns of the treated MWCNT and the synthesized nanocomposite show the obvious peaks that refer to the formation of nano MnO2 onto MWCNT. The treated MWCNT shows a sharp peak at around 26° and a broad weak peaks at around 43° and 53° (Fig. 1a), which can be well assigned to the (0 0 2), (1 0 0) and (0 0 4) planes of graphite carbon [17]. Fig. 1b showed the two typical broadening patterns of the MnO2/MWCNT samples of 2 at around 65.5 that can be ascribed the crystal planes of (0 2 0) in γ-MnO2 [18,19]. And it is obvious after deposition of MnO2, all surface of treated MWCNT completely covered by the nanoflakes of MnO2 (Fig. 2). The deposited MnO2 onto treated MWCNT exhibits a core-shell structure composed of many nanoflakes with a highly porous structure. [15,20]
The adsorbed amount of chromium (III) onto the MnO2/MWCNT nanocomposite were calculated by the following equations [21,22]:

Where C0 is the initial concentration (mg/L),Ct is the amount of concentration (mg/L) at any time t and Ce the amount of concentration (mg/L) in equilibrium, V is solution volume (L); and M is MnO2/MWCNT nanocomposite mass (g).
Results and Discussion
pH optimization
In this research, the pH of solution influences the distribution of active sites on the surface of MnO2/MWCNT, to find out the effects of solution pH on adsorption capacity; Cr(III) adsorption was examined by adding different amounts of 0.1 M HCl or 0.1 M NaOH to obtain different pHs. In Fig. 3, it can be described that the amount of Cr(III) adsorbed on MnO2/MWCNT increased by increasing pH at 5, so we carry out the rest of experiment in this condition.
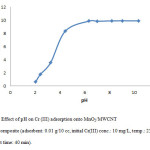 |
Figure 3: Effect of pH on Cr (III) adsorption onto MnO2/MWCNT nanocomposite (adsorbent: 0.01 g/10 cc, initial Cr(III) conc.: 10 mg/L, temp.: 25°C, contact time: 40 min). Click here to View figure |
Dose of adsorbent
In the next step, we identify the best adsorbent dosage, as we declare the less amount of adsorbent is also much profitable. Then we were measuring this parameter in 5 different dosage. We choose the adsorbent doses in 0.001g, 0.005 g, 0.008 g, 0.01 g, 0.015 g and we realize in a very low amount of adsorbent can reach to high level of removal too. Therefore we continue the rest of the reaction by using about 0.005 g of adsorbent. These results indicate that MnO2/MWCNT nanocomposite is promising candidate as highly effective adsorbent (Fig. 4).
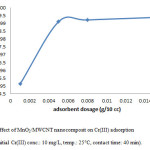 |
Figure 4: Effect of MnO2/MWCNT nanocomposit on Cr(III) adsorption (pH: 5, initial Cr(III) conc.: 10 mg/L, temp.: 25°C, contact time: 40 min). Click here to View figure |
Contact time
To demonstrate the optimum contact time 0.005 g of MnO2/MWCNT nanocomposite was added to 10 ml of Cr(III) solution with concentration of 10 mg/L at 25° C. the pH of solution was 5 and we carried out this parameter in four different period time, samples were taken in 10 min, 25 min, 40 min (Fig. 5). So we find out even at the initial period contact time, the removal of Cr(III) was very fast, but by increasing contact time we reach to the much more removal of Cr(III).
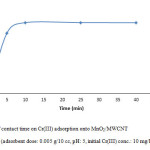 |
Figure 5: Effect of contact time on Cr(III) adsorption onto MnO2/MWCNT nanocomposite (adsorbent dose: 0.005 g/10 cc, pH: 5, initial Cr(III) conc.: 10 mg/L, temp.: 25°C). Click here to View figure |
Effect of temperature
To identify the effect of temperature, 0.005 g of MnO2/MWCNT nanocomposite was added to 10 ml of Cr(III) solution with concentration of 10 mg/L at PH 5 and the experiments were carried out at temperatures of 35, 45, 55 °C. according to our investigations, the adsorbent amount of Cr(III) on MnO2/MWCNT nanocomposite decreases with the increasing temperature. Fig. 6 shows the effect of temperature on removal of Cr(III).
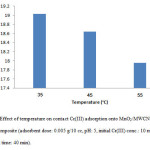 |
Figure 6: Effect of temperature on contact Cr(III) adsorption onto MnO2/MWCNT nanocomposite (adsorbent dose: 0.005 g/10 cc, pH: 5, initial Cr(III) conc.: 10 mg/L, contact time: 40 min). Click here to View figure |
Influence of initial ion concentration on Cr(III) adsorption
The initial ion concentration provide significant driving force in adsorption process, it can noted that the adsorption percent of Cr(III) decrease by increasing chromium concentration (Fig. 7, Fig. 8). We carried out our experiment in 10 to 50 mg/L.
Adsorption isotherm study
In order to investigate the mechanism of Cr(III) adsorption on adsorbent and for declaring the chemical equilibrium between Cr(III) and adsorbent, Langmuir and Fruendlich and also Temkin isotherms were studied to describe equilibrium. Adsorption isotherm studies were carried out with various initial Cr(III) concentrations ranging from 10 to 50 mg/L.
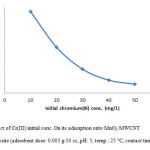 |
Figure 7: Effect of Cr(III) initial conc. On its adsorption onto MnO2/MWCNT nanocomposite (adsorbent dose: 0.005 g/10 cc, pH: 5, temp.: 25 °C, contact time: 40 min). Click here to View figure |
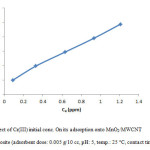 |
Figure 8: Effect of Cr(III) initial conc. On its adsorption onto MnO2/MWCNT nanocomposite (adsorbent dose: 0.005 g/10 cc, pH: 5, temp.: 25 °C, contact time: 40 min). Click here to View figure |
The linearized form of Langmuir adsorption isotherm equation is as follow:
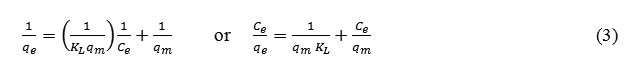
The linearized form of Freundlich adsorption isotherm equation:

The linearized form of Temkin adsorption isotherm equation:
![]()
Where Ce (mg/L) is the equilibrium concentration of Cr(III), qe (mg/L) is the amount of Cr(III) adsorbed at equilibrium, qm (mg/g) is the maximum adsorption at monolayer and KL (L/mg) is the Langmuir constant including the affinity of binding sites. KF[(mg/g)(L/mg)1/n] and n are Freundlich constants indicating adsorption capacity and intensity, respectively.
KT (L/g) and B1 are the Temkin constants (KT is the equilibrium binding constant and B1 is related to the heat of adsorption). The amounts of Langmuir, Freundlich and Temkin parameters were calculated from the slope and intercept of linear plots of 1/qe versus 1/Ce, Ln qe versus Ln Ce and qe versus Ln Ce, respectively [23,24,25].
Further we display the adsorption isotherms plots (Fig. 9).
a)
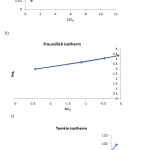 |
Figure 9: Plots of linearzed Langmuir (a), Freundlich (b), and Temkin (c) adsorption isotherms. Click here to View figure |
It can be concluded from isotherm plots that the reaction process is much more compatible by Freundlich isotherm than others.
The isotherm constants also listed in table 1.
Table 1: Isotherm parameters for Cr(III) adsorption onto MnO2/MWCNT nanocomposite (adsorbent dose: 0.005 g/10 mL, pH: 5, temp: 25°C, contact time: 40 min)
|
Langmuir model |
Freundlich model |
| qm (mg g-1) R2 | KF n R2 |
| 99.01 2.73 0.9727 | 78.15 1.74 0.9958 |
In this work, also the thermodynamic parameters were calculated to evaluate the nature of adsorption process [26,27]. Thermodynamic parameters such as Gibss free energy change (ΔG0, KJ/mol), enthalpy change (ΔH0, KJ/mol) and entropy change (ΔS0, J/mol.k) can be estimated as following:

Where Kc is the equilibrium constant of the adsorption process, C0 and Ce are the initial and equilibrium concentration of Cr(III). In table 2 we also demonstrate thermodynamic parameters.
Table 2. Thermodynamic parameters for adsorption of Cr(III) on MnO2/MWCNT
|
t (°C) |
C0 (mg/L) |
Ce (mg/L) |
K |
lnK |
1/T (K-1) |
∆G0 (kJ/mol) |
∆H0 (kJ/mol) |
∆S0 (J/mol K) |
|
35 |
10 |
0.487 |
19.53 |
2.97 |
0.0032 |
-7.61 |
-33.26 |
-81.59 |
|
45 |
10 |
0.679 |
13.72 |
2.62 |
0.0031 |
-6.93 |
-33.26 |
-81.59 |
|
55 |
10 |
1.022 |
8.78 |
2.17 |
0.0030 |
-5.92 |
-33.26 |
-81.59 |
We can conclude this reaction is exothermic and spontaneous because Cr(III) adsorption decrease by increasing temperature. The value of ∆S0 (J/mol K) indicates Cr(III) molecules reach to the arrangement in this adsorption.
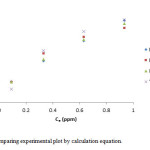 |
Figure 10: Comparing experimental plot by calculation equation. Click here to View figure |
Conclusion
In this work we investigated the optimization conditions for removal of Cr(III) ions by MnO2/MWCNT nanocomposite that we reach to a fairly good results. We investigated the adsorption in a wide range of pH to identify the best range. So we found that the pH = 5 is better. Therefore we did our measurements in this pH. We also investigated the best dosage of adsorbent, contact time, and also various concentrations have been studied. In lower concentration we got better results, and then we estimated thermodynamic parameters. As we found the studied adsorption was exothermic and spontaneously. Also we compare our experimental results with our calculation results. We concluded, the our results are better comparable with Freundlich isotherm.
References
- Babu, B. V; Gupta, S,; Removal of Cr(VI) from wastewater using activated tamarind seeds as an adsorbents [J]. Journal of Environmental Engineering and Science, 2008, 7(5): 553-557.
- Mohanty, M,; Patra, H. K,; Attenuation of chromium toxicity by bioremediation technology [J]. Reviews of Environmental Contamination and Toxicology, 2011, 210: 1-34.
- Lv X, Xu J, jiang G,Tang J, Xu X: Highly active nanoscale Zero-valent iron (nZVI)-Fe <sub> 3</sub> O <sub> 4</sub> nanocomposites for the removal of chromium (VI) from aqueous solutions. J colloid interface Sci 2012, 369:460-469
- Luo C, Tian Z,Yang B, Zhang L, Yan S: Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal. Chem Eng J 2013, 234:256-265.
- Lv X, Xu J, jiang G, Xu X: Removal of chromium (IV) from wastewater by nanoscale zero-valent iron particles supported on multi-walled carbon nanotubes. Chemosphere 2011, 85:1204-1209.
- Wu L, Liao L,Lv G, Qin F, He Y, Wang X: micro-electrolysis of Cr(VI) in the nanoscale zero-valent iron loaded activated carbon. J Hazard mater 2013, 254:277-283
- Jung C, Heo J, Han J, Her N, Lee S-J, Oh J, Ryu J, Yoon Y: Hexavalent Chromium removal by various adsorbents: powdered activated carbon, chitosan and single/multi walled carbon nanotubes. Sep Purif Technol 2013, 106:63-71
- Remero-Gonzale, J,; Walton, J. C,; Peralta-Videa J. R,; Rodregoez, E,; Romero, J,; Gardea-Torresdey, J. L,; modeling the adsorption of Cr(III) from aqueous solution of onto Agave lechuguilla biomass: Study of the advective and dispersive transport [J]. journal of Hazardous materials, 2009, 161(1): 360-365
- Kumar, PA,; Ray, M,; Chakraborty, S,; Adsorption behavior of trivalent chromium on amine-based polymer aniline formaldehyde condensate [J]. Chemical Engineering Journal, 2009(1/3): 340-347.
- Narayanan, V,; Ganesan, M,; use of adsorption using granular activated carbon (GAC) for the enhancement of removal of chromium from synthetic wastewater by electrocoagulation [J]. Journal of Hazardous Materials, 2009, 161: 575-580.
- Meltaz, H.F; Carasek, E. Chromium speciation and preconcentration using zirconium(IV) and zirconium(IV) phosphate chemically immobilized onto silica gel surface using a flow system and FAAS. Talanta 2005, 65, 337-542.
- Liang, P.; Shi, T. Q.; Lu, H.B.; Jiang, Z.C.; Hu, B. Speciation of Cr(III) and Cr(IV) by nanometer titanium dioxide micro-column and inductively coupled plasma atomic emission spectrometry. Spectrochim. Acta Part B 2003, 58, 1709-1714.
- Beni, A.; Karosi, R.; Posta, J. Speciation of hexavalent chromium in waters by liquid-liquide extraction and GFAAS determination. Microchem. J. 2007, 103-108.
- Zhang, N.; Jibrin, S.S; He, M.; Hu, B. Chromium(III)-imprinted silica gel for speciation analysis of Chromium in environmental water samples with ICP-MS detection. Talanta 2008, 75, 536-543
- Zheng, H.; Wang, J.; Y. C. Ma, Jia,; In-situ synthetize multi-walled carbon nanotubes@MnO2 nanoflake core-shell structured materials for supercapacitors, Journal of Power Sources 216 (2012) 508-514.
- Wang, H.J.; Peng, C.; Peng, F.; Yu, H.; Yang, J,; Facile synthesis of MnO2/CNT nanocomposite and its electrochemical performance for supercapacitors, Materials Science and Engineering B 176 (2011) 1037-1078
- Wang, H.J.; Zhu, L.L.; Peng, S.; Pwng, F.; Yu, H.; Yang, J,; High efficient conversion of cellulose to polyols with Ru/CNTs as Catalyst, Renewable Energy 37 (2012) 192-196
- Xie, X.; Gao, L.; Characterization of a manganese dioxide/carbon nanotube composite fabricated using an in situ coating method, Carbon 45 (2007) 2365-2373.
- Julien, C. M.; Massot, M.; Poinsignon, C.; Lattice vibration of manganes oxides: Part I. Periodic structures, Spectrochimica Acta Part A 60 (2004) 689-700.
- Xia, H.; Wang, Y.; Lin, J.; Lu, L.; Hydrothermal synthesis of MnO2/CNT nanocomposite with a CNT core/porous MnO2 sheath hierarchy architecture for supercapacitors, Nanoscale Research Letters 7 (2012) 1-10.
- El-Sheikh, A. H.; Sweileh, J. A.; Al-Degs, Y. S.; Effect of dimensions of multi-walled carbon nanotubes on its enrichment efficiency of metal ions from environmental waters, Anal. Chim. Acta 604 (2007) 119-126.
- Jun, Hu,; Dadong, Shao,; Changlun, Chen,; Guodong, Sheng,; Xuemei, Ren,; Xiangke, Wang,; Removal of 1-naphthylamine from aqueous solution by multiwall carbon nanotubes/iron oxides/cyclodextrin composite, Journal of Hazardous Materials 185 (2011) 463-471.
- Langmuir, I.; The constitution and fundamental properties of solids and liquids, Jornal of American Chemical Society 38 (1916) 2221-2295
- Freundlich, H. M. F.; Uber die adsorption in losungen. Zeitschrif Fur. Physikalische Chemie A, 57 (1906) 385-470
- Temkin, M.; Pyzhev, V.; Kinetics of the Synthesis of Ammonia on Promoted Iron Catalysts. Acta Physicochemical U.R.S.S 13 (1940) 851-867
- Sarin, V.; Pant K K.; Removal of Chromium from industrial waste by using eucalyptuse bark J. Bioresourse Technology 97 (1) (2006) 15-20
- Sarin, V.; Singh, T s.; Pant, K K.; Thermodynamic and breakthrough column studies for selective sorption of chromium from industrial effluents on activated eucalyptus bark [J]. Bioresource Technology, 97(16) (2006) 1986−1993.

This work is licensed under a Creative Commons Attribution 4.0 International License.









