Synthesis of Phenolic-Based Resist Materials for Photolithography
Sutikno*, Muhammad Lukman Hakim and Sugianto
Physics Department, Faculty of Mathematics and Natural Sciences, Semarang State University (Unnes), Sekaran Street, Gunungpati Semarang, 50229, Indonesia. Corresponding Author Email: smadnasri@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320117
Article Received on :
Article Accepted on :
Article Published : 10 Mar 2016
Phenol-basedphotoresist for photolithography application is successfully developed in this research namely by mixing phenolic resin with ethanol solvent and sodium acetate 3-hydrate 30% of phenolic resin mass. Novolac phenolic resin is made by mixing formaldehyde and phenol in mol ratio 2,8:1 catalyzed using NaOH. Phenolic resin is made at heating temperature of 85°C and stirring rotation speed of 1000 rpm. Photoresist thin film is spincoated on the glass substrate and prebaked on the hotplate at 95°C for 60 s. The research result shows that microstructures of resist film which are manufactured of resol phenolic resin seem more homogeneous than that of novolac phenolic resin. The maximum absorbance is in the wavelength range of 380-500 nm, its density and viscosity are 1,41 g/ml and 198 centipoise, respectively.
KEYWORDS:Novolac; Phenolic; Photoresist; Photolitography; Spincoating
Download this article as:| Copy the following to cite this article: Sutikno, Hakim M. L, Sugianto. Synthesis of Phenolic-Based Resist Materials for Photolithography. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Sutikno, Hakim M. L, Sugianto. Synthesis of Phenolic-Based Resist Materials for Photolithography. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14851 |
Introduction
The Indonesian goverment attempts in developing industries have been still facing no warranty of raw material supplies. Especially for microelectronic industry, the supply sustainability of raw materials are no assurrance so that it becomes a serious problem for the local microelectronic industry development. The crucial step here is how to ensure the avaibility of raw materials for microelectronics industries. The potency of Indonesia natural resources poses the second rank after Brazil either for biodiversity or minerals resources. Many organic materials which can be converted into raw materials for microelectronic industry, for example natural phenol is available to manufacture photoresist which is applicable for photolihography.
In recent years, the synthesis of solar sensitive polymer has been developed for photolithography. Some polymers sensitive to light are applied in microlithography, printing material, liquid crystal and non-linear optical. These polymer properties are good solubility, good film forming capability, high photosensitivity, un-soluble in solvent after crosslinking, high thermal stability and resistance to plasma and etching agents [1].
The resist synthesis for microlithography is necessary developed, because the lithography process is a key to produce electrical circuits in small dimension and to shrink component size in microlectronic device. The decrease of device size increases it’s capability and decrease the production cost [2].
To increase density of integrated circuit (IC), feature size of patterns made using lithography will be smaller. In recent years, organic materials are considered very promising to manufacture photoresist because they have good photothermal sensitivity, long lifetime, processable in vacuum, clear border line between exposed and un-exposed area, light molecule weight and stronger chemical bonding [3].
The photoresist is multicomponent system which consist of photoresist polymer, photoacid generator (PAG), and aditive [4]. It is an important chemical in semiconductor processing, liquid crystal display (LCD) and printing process [5]. In the manufacturing process of semiconductor device, photoresist is used as mask [6]. The photoresist composite is also developed for electrode photopatterning which available for biochip [7].
One of most important polymers is formaldehyde phenol resin polymer. Formaldehyde phenol resin is synthetic polymer of reaction result of phenol and formaldehyde. The phenolic resin is main material to produce circuit patterns [8]. The formaldehyde phenol is the first synthetic resin which used in commercial industries either plastic or paint industry. The condensation reaction of phenolic on different two conditions includes resol and novolac [9]. The polymer which often used to manufacture photoresist is novolac resin namely formaldehyde phenolic polymer with acid as catalyst [10].
The photoresist performance in microplithography depends on the used polymer. Many reasearchs are focused ininvestigating polymer microstructuresto achieve high resolution [10]. The polymer also functions to control solution viscosity. Another function is to support film forming and to increase mechanical properties of resulted photoresist liquid [11].The photoresist appplications in microelectronic industry range very wide area. The viscosity of polymer can be optimised by variating mol fraction offormaldehyde and phenol and reaction duration. The different polymer viscosity in manufacturing photoresist will produce photoresist with different viscosities, so that in the film forming will lead to different microstructures and the different absorbances.
Materials and Method
The used equipments include Ohauss balance, pipette, heated magnetic stirrer, beaker glass, measuring glass, thermometer, spincoater, scopeman digital CCD microscope MS-804, spectrophotometer (ocean optic Vis-NIR USB 4000), and brookfield dial viscometer.The materials used in this research consist ofpreparate glass, phenol (C6H5OH), formaldehyde (CH2O), 4-tert-butylphenol (C10H14O), natrium hydroxide (NaOH), acid sulphate (H2SO4), ethanol (C2H5OH), and sodium acetate trihydrate (Na-C2-H3-O2.3H2O).
To make resin phenolic resol polymer, phenol (0,8 mol phenol, 0,2 mol and 0,2 mol 4-tert-butylphenol) and 2,8 mol formaldehyde are mixed. On the other hand, resin phenolic novolac is made in two composition, first one contains phenol (0,8 mol phenol, 0,2 mol 4-tert-butylphenol) and 0,8 mol formaldehyde, and another one contains phenol (0,8 mol phenol, 0,2 mol 4-tert-butylphenol) and 0,75 mol formaldehyde. To make resin phenolic novolac and resin phenolic resol were used sulphate acid and natrium hidroxyde, respectively, by through mixing process using heated magnetic stirrer at speed of 1000 rpm and temperature of 85oC.
The photoresist is made through mixing of resin phenolic polymer with ethanol amount of 20% of mass total of polymer and sodium acetate 3-hydrate (photosensitive material) amount of 30% of resin phenolic mass using heated magnetic stirrer at constant speed 100 rpm up to it’s temperature achieved 85oC. The photoresist thin films were spincoated on preparate glasses. The photoresist liquid was dropped on the preparate glasses at constant speed for 60 seconds.
The microstructures of photoresist thin films were observed using scopeman digital CCD microscope MS-804 and their absorbances were measured in the wavelength range of 350-1000 nm using spectrometer (ocean optic Vis-NIR USB 4000). The viscosity of photoresist liquid is measured using brookfield dial viscometer and it’s densities were measured using ratio of mass and volume for each sample.
Results and Discussion
In Figure 1, surface of photoresist thin film seems homogeneous, nevertheless there has been seem a dot in that surface which shows an impurity. Figure 2 is taken in magnification of 2400x. In general, the surface microstructures of resin phenolic resol polymer show smooth and homogeneous surfaces.
 |
Figure 1: Microstructure of photoresist thin film with mol ratio of formaldehyde and phenol equals to 2,8 and 1 and catalyst reaction during 35 minutes. Click here to View figure |
 |
Figure 2: The surface structure of polymer photoresist thin film with mol ratio of formaldehyde and phenol equals to 2,8 and 1 in catalyst reaction for: (a) 37,5 (b) 40 (c) 42,5 minutes. |
The photoresist thin films of resin phenolic novolac have different surface microstructures as shown in Figure 3. Figure 3a appears that the resulted photoresist thin films have rough surface structure and inhomogeneous. The same condition is found in Figure 3b. The catalyst used here is sulphate acid. The surface structure of Figure 3 looks more homogeneous and smoother than those of Figure 3a and 3b. As the durationof the catalyst (sulphate acid) reaction increases in making photoresist novolac polymer the homogenity increases as well.
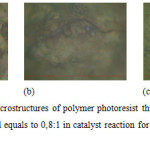 |
Figure 3: The surface microstructures of polymer photoresist thin films with mol ratio of formaldehyde and phenol equals to 0,8:1 in catalyst reaction for: (a) 37,5 (b) 40 (c) 42,5 minutes. |
Figure 4a looks that the surface structure of photoresist thin film is rough and inhomogeneous. Figure 4b is more homogeneous and smoother than that of Figure 4a. Figure 4c has smoother surface structure and more homogeneous than those of Figures 4a and 4b. The longer reaction of catalyst leads to more homogeneous liquid. It is found that the photoresist thin films of resin phenolic resol have smoother and more homogeneous surface structures than those of resin phenolic novolac.
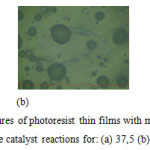 |
Figure 4: The surface structures of photoresist thin films with mol ratio of formaldehyde and phenol equals to 0,75:1 in the catalyst reactions for: (a) 37,5 (b) 40 and (c) 42,5 minutes. |
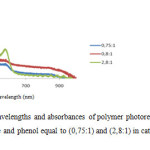 |
Figure 5: Graph of wavelengths and absorbances of polymer photoresist thin films with mol ratios of formaldehyde and phenol equal to (0,75:1) and (2,8:1) in catalyst reaction for 37,5 minutes. |
The maximum absorbances of polymer photoresist range 380-500 nm as shown in Figure 5. The mol ratios of formaldehyde and phenol increase the absorbances increase as well. The relationship between reaction periods with absorbances is shown in Figure 6.
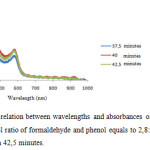 |
Figure 6: Graph of relation between wavelengths and absorbances of thin films of polymer photoresist with mol ratio of formaldehyde and phenol equals to 2,8:1 in catalyst reaction during 37,5, 40, dan 42,5 minutes. |
In Figure 6 seems no relationship is found between reaction periods with absorbaces of resulted photoresist thin films.
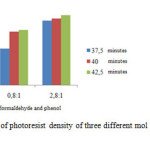 |
Figure 7: The bar chart of photoresist density of three different mol ratio for 37,7, 40 and 42,5 minutes. |
In Figure 6 seems no relationship is found between reaction periods with absorbaces of resulted photoresist thin films. As the mol ratio of formaldehyde and phenol increases the density of photoresist increases as well. The maximum density of maunfactures photoresist achieves 1,43 g/ml. For comparison, Photoresist S1800 series, a commercial photoresist, have density of 1,02 g/ml [12]. The data of measured density of manufactured photoresists are shown in Table 1.
Table 1: Viscosities of polymer photoresists manufactured of phenolic resin novolac.
|
Read value on viscosimeter |
Multiplier factor |
Viscosities (centipoise) |
|
16 |
2 |
32 |
|
16,5 |
2 |
33 |
|
17 |
2 |
34 |
Table 2: Viscosities of polymer photoresists of resin novolac resol.
|
Read value on viscosimeter |
Multiplier factor |
Viscosities (centipoise) |
|
99,5 |
2 |
199 |
|
99 |
2 |
198 |
|
99,5 |
2 |
199 |
Based on the Tables 1 and 2, the photoresist of resin novolac polymer has average viscosity of 33 centipoise and the polymer photoresist of resin phenolic resol has average viscosity of 198,6 centipoise. The samples which are measured their densities are of polymer photoresist with mol ratio of formaldehyde and phenol equals to 0,75:1. The used resin phenolic novolac is made of acidsulphate, formaldehyde and 4-tert butylphenol through mixing for 40 minutes.Based on the measurement results, the viscosity of polymer photoresist of resin phenolic resol is higher than that of resin phenolic novolac.
Conclusion
In conclusion, the increase of mol ratio of formaldehyde and phenol and the increase of catalyst reaction duration have made the surface structures of photoresists more homogeneous and smoother. The photoresist absorbances are not influenced by catalyst reaction duration. The thin film absorbances of resin phenolic resol are higher than those of resin novolac. The wavelength of maximum absorbances are in the range of 380-500 nm. The mol ratio of formaldehyde and phenol increases the photoresist absorbance increases as well. The density of photoresist of resin phenolic resol is higher than that of resin novolac.
Acknowledgment
This research was supported by Ministry of Research, Techology and Higher Education, Republic Indonesia through Hibah Bersaing (Competitive Grant).
References
- Rehab, A. Eur. Polym. J. 1998, 34, 1845-1855
CrossRef - Aronson, C.L.; Beloskur, D.; Frampton, I.S.; McKie, J.; Montbriand, P. Polym. Bul. 2004, 52, 409-419
CrossRef - Xi, H.; Liu, Q; Guo, S. Mater. Let. 2012, 80,72–74
CrossRef - Prabhu, V.M.; Sambasivan, S.; Fischer, D.; Sundberg, L.K.; Allen., R.D. Appl. Surf. Sci. 253, 2006, 1010–1014
CrossRef - Kim, Y.H.; An, E.S.; Park, S.Y., Lee, J.O.; Kim, J.H.; Song, B.K. J. Mol. Catal. B-Enzym. 2007, 44, 149–154
CrossRef - Saito, R. Ichinohe, Y.; Kudo, M. Appl. Surf. Sci. 1999, 142, 460–464
CrossRef - Benlarbi, M.; Blum, L.J.; Marquette, C.A. Biosens. Bioelectron. 2012, 38220–225
- Wang, M.; Yuan, Z.; Cheng, S.; Leitch, M.; Xu, C.C. J. Appl.Polym. Sci. 2010, 118, 1191–1197
- Ku, H.; Jacobson, W.; Trada, M.; Cardona, F.; Rogers, D. J. Compos. Mater. 2008, 42, 2783
CrossRef - Sharma, M., Naik, A.A., Raghunathan, P., Eswaran, S.V., J. Chem. Sci. 2012, 124, 395–401
CrossRef - Feiring, A.E.; Crawford, M.K.; Farnham, W.B.; Feldman, J.; French, R.H.; Leffew, K.W.; Petrov, V.A.; Schadt III, F.L.;Wheland, R.C.; Zumsteg, F.C. J. Fluorine Chem. 2003, 122, 11–16
CrossRef - O’Neill, F.T.; Sheridan, J.T. Optik 2002, 113, 391–404
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









