Synthesis and Antioxidant Ability of New 5-amino-1,2,4-Triazole Derivatives Containing 2,6-dimethoxyphenol
Dhuha Faruk. Hussain*
Department of Chemistry, Ibn Al-haitham, University of Baghdad, Baghdad 61023, Iraq Corresponding author E-mail: dhuha_faruk@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320162
Article Received on :
Article Accepted on :
Article Published : 02 Feb 2016
4-amino-3-(4-(((4-hydroxy-3,5dimethoxybenzyl)oxy)methyl)phenyl)-1,2,4-triazole-5-thione was synthesized by to method the first one from melt reaction of 4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzoic acid with Thiocarbonyldihydrazide, the second method from convert the corresponded acid hydrazide to potassium 2-(4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzoyl)hydrazinecarbodithioate salt then react with hydrazine hydrate. Newly Schiff base (7a-7f) were synthesized from reaction the 4-amino-1,2,4-triazol with substituted hydroxybenzaldehyde. The resulting compounds were characterized by IR, 1H-NMR, 13C-NMR, and HRMS data. 2,2-Diphenyl-1-picrylhydrazide (DPPH) and ferric reducing antioxidant power (FRAP) assays were used to screened the antioxidant properties of the synthesized compounds. Compounds 7d , 7e and 7f exhibited significant free-radical scavenging ability in both assays.
KEYWORDS:2;6-dimethoxyphenol; 4-amino-1,2,4-triazole; Schiff base; antioxidant; DPPH; FRAP
Download this article as:| Copy the following to cite this article: Hussain D. F. Synthesis and Antioxidant Ability of New 5-amino-1,2,4-Triazole Derivatives Containing 2,6-dimethoxyphenol. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Hussain D. F. Synthesis and Antioxidant Ability of New 5-amino-1,2,4-Triazole Derivatives Containing 2,6-dimethoxyphenol. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13778 |
Introduction
The reactive oxygen species (ROS) and other related free radicals species are witty to react either directly or indirectly, to damage all biomolecules. This damage can cause many diseases 1, such as inflammatory2, cancers3, degenerative4 and chronic diseases5. The antioxidant compounds are the most important species which can inhibit the oxidative stress in biological system and prevent any free radicals damage. The phenolic compounds are one of the significant antioxidant type, as well reported it possess broad biological activity such as anti inflammatory6 , anticancer7 and gastroprotective8.
Generally, antioxidants compounds donate protons to become stable free radicals. This stability increases with the extent of delocalization and enhances the antioxidant ability9, 10 . As such, many synthesized compounds containing long chain resonance exhibited significant antioxidant activity. Furthermore, the compounds which can be considered a strong antioxidant usually possess common structural features. They often own multiple phenolic hydroxyl groups like flavonoids 11 or which have full conjugation π system like carotenoids12. Moreover, exhibited substituted groups might influence on the scavenging ability. This indicates the existence of a close relationship between the chemical structure and the ability to scavenge free radicals.
Derivatives of 1,2,4-triazole exhibited various types of biological activities e.g. Antimicrobial13, antiviral,14, 15 inhibitors of Methionine Aminopeptidase-2,16 Anhydrase inhibitors,17 anti-cancer18, anti-inflammatory,19 inhibitors of the HIV-1,20 analgesic 21 and antioxidant 22.As well the compounds of Schiff base derivatives displayed broader properties and applications such as nonlinear optical devices 23 liquid crystalline 24, 25, dyes 26, biological 27-29 and pharmaceutical 30 applications.
The 2,6-dimethoxyphenol derivatives won wide attention in recent years as antioxidants31, 32 . In this work we presented the synthesis of new 4-((arylidine)amino)-3-(4-(((4-hydroxy-3,5-dimethoxybenzyl) oxy)methyl)phenyl)-1,2,4-triazole-5-thione (7a-7f) as new antioxidant material.
Material and Method
Chemistry
The IR spectra were obtained with a Perkin Elmer 400 Fourier Transform Infrared (FTIR) Spectrometer. 1H and 13C-NMR spectra were recorded at Joel Lambda spectrometers at 400 MHz) UM, Malaysia). DMSO-d6 were used as solvents with TMS as the internal standard. The mass spectra were recorded using an Agilent 5975 system for EI/MS and a Finnigan TSQ7000 for HREIMs (NUS, Singapore). For UV spectroscopy, a Power Wave X340 (BIO-TEK Instruments, Inc., Winooski, VT, USA) was used to record the FRAP and DPPH. Melting points were measured on a Sturt-SMP10 melting point apparatus in open-end capillary tubes. Flash column chromatography on silica gel 60 (230–400 mesh, E. Merck) was employed. General grade solvents and reagents were purchased from commercial suppliers and used without further purification.
4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzoic acid 4
This compound was synthesized according to the procedure described by K. F. Ali 33.Recrystallized with ethanol to obtain white microcrystals. Yield 60 %, Mp 150-152 ̊C [ lit. 152-154 ̊C 33], IR (KBr) vmax 3354 (OH), 3046 (CHAr), 2972, 2893 (CHaliphatic), 1676 (C=O) 1585 (C=C), 1180 (Ar-O-C), cm-1. 1H-NMR (400MHz, CDCl3): 3.87(6H, s, 2×OCH3), 4.33 (2H, s, OCH2), 4.38 (2H, s, OCH2), 6.66 (s, 2H, H-3), 7.65 ( 2H, d, J 8.2, H-8),8.12( 2H, d, J 8.0, H-9). 9.15 (1H, bs, OH), 13C-NMR (100 MHz, CDCl3): 56.11(2C, OCH3), 71.22(1C, CH2OCH2), 72.77 (1C, CH2OCH2), 109.05(2C, CH), 128.84(2C,CH), 129.37(1C), 131.21(2C, CH), 134.23 (1C), 139.65 (1C), 142.13(1C) 152.01 (2C), 169.58 (1C, C=O) HREIMs m/z = 318.1100 [M˙+] (calc. for C17H18O6, 318.1103).
4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzohydrazide 5
This compound was synthesized according to the procedure described by K. F. Ali 33.The crude was recrystallized from ethanol to give white solid. Yield 87%, Mp 94-96 ̊ C [ lit. 92-94 ̊C 33] , IR (KBr) vmax 3422 (OH phenol), 3308, 3218 (NH, NH2), 3037 (CHAr), 2975 -2885 (CHaliphatic), 1661 (C=O), 1585 (C=C), 1151(Ar-O-C), cm-1. 1H-NMR (400MHz, DMSO-d6): 3.71 (6H, s, 2× OCH3), 3.93 (2H, s, OCH2), 4.09 (2H, s, OCH2), 4.63 (bs, 2H, NH2) 6.52 (s, 2H, H-3), 7.67 ( 2H, d, J 8.1, H-8), 8.22( 2H, d, J 7.92, H-9), 8.863 (1H, bs, CONH), 9.21(1H, bs, OH), 13C-NMR (100 MHz, DMSO-d6): 56.3 (2C, OCH3), 71.14 (1C, CH2OCH2), 72.60 (1C, CH2OCH2), 106.88(2C, CH), 129.11(2C,CH), 128.92(1C), 130.01(2C, CH), 133.42(1C), 139.04(1C), 141.55(1C) 152.01 (2C), 166.15(C=O). HREIMs m/z = 332.1369 [M˙+] (calc. for C17H20N2O5, 332.1372)
General synthesis of 4-amino-3-(4-(((4-hydroxy-3,5dimethoxybenzyl)oxy)methyl)phenyl) -1,2,4-triazole-5-thione. 6
Method A
4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzoic acid (3.2gm 10.05,mmol) and Thiocar-bonyldihydrazide (1.17 gm , 11 mmol) was mixed then grinded after that the mixture was transferred to conical flask and heated until melt at 160-165 ˚C for three hour. The mixture left to cool to ambient temperature.The crude was boiled with (2×20 mL) dichloromethane then filtrated. The organic combined evaporated under reduced pressure. The crude precipitated was recrystallized from ethanol to afford white crystals. Yield 51% Mp 68-70 ˚C,
Method B
To a solution of 4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzohydrazide (3.33 g, 10 mmol) in xx mL absolute ethanol was added. Carbon disulphide (0.92 g, 12 mmol) was added and potassium hydroxide (0.56 g, 10 mmol) at room temperature. The mixture was stirred for 24 h, then 25 mL dry ether was added and allow to stir for another 2 h. The precipitated was filtrated and washed with dry ether. The product was dried at 80 °C to give white solid of potassium 2-(4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzoyl)hydrazinecarbodithioate salt (4 g,9 mmol). The resulting product was dissolved in excess of hydrazine hydrate 80%. The mixture was heated and refluxed for 7 h then cooled and poured into ice water. The pH was adjusted to 5 by using 10% HCl. The precipitate was filtrated, washed with water, dried and recrystallized from ethanol to obtain yield 68% of white crystals. Mp 68-70.
IR (KBr) vmax 3507 (OH phenol), 3410, 3330and 3170 (NH, NH2), 3013 (CHAr), 2995 -2887 (CHaliphatic), 1628 (C=N), 1585 (C=C), 1362(C-N), 1245(C=S), 1201(Ar-O-C), cm-1. 1H-NMR (400MHz, DMSO-d6): 3.82 (6H, s, 2× OCH3), 3.89 (2H, s, OCH2), 3.98 (2H, s, OCH2), 6.13 (bs, 2H, NH2) 6.58 (s, 2H, H-3), 7.69 (2H, d, J 8.0, H-8), 8.25( 2H, d, J 7.82, H-9), 9.27(1H, bs, OH),14.03(1H, bs, NH). 13C-NMR (100 MHz, DMSO-d6): 57.21, (2C, OCH3), 72.04 (1C, CH2OCH2), 72.69 (1C, CH2OCH2), 107.17(2C, CH), 128.78(2C,CH), 129.90(1C), 131.10 (2C, CH), 133.72(1C), 138.86 (1C), 142.53(1C) 149.54 (1C,C=N), 152.01 (2C), 166.61(C=S). HREIMs m/z = 388.1202 [M˙+] (calc. for C18H20N4O4S, 388.1205)
General synthesis of 4-((arylidine)amino)-3-(4-(((4-hydroxy-3,5-dimethoxybenzyl) oxy)methyl)phenyl)-1,2,4-triazole-5-thione 7a-7f
aryl aldehyde (0.51mmol) was added to a stirred solution of 4-amino-3-(4-(((4-hydroxy-3,5dimethoxybenzyl)oxy)methyl)phenyl) -1,2,4-triazole-5-thione (0.2 gm, 0.51mmol) in 5 mL glacial acetic acid and the mixture was refluxed for 9h. After cooling the mixture was poured into 50 mL crushed ice. the precipitate collected, washed with cold distilled water and recrystallized from suitable solvent.
4-((4-hydroxybenzylidene)amino)-3-(4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy) methyl)phenyl)-1,2,4-triazole-5-thione 7a
Recrystallized from methanol to give pale yellow precipitate.Yield 83%, Mp 115-117 ˚C IR (KBr) vmax, 3498(OH phenol), 3152(NH), 3062(CHAromatic), 1623 (CH=N), 1607 (C=Ntriazole), 1995, 1985(C=C), 1247(C=S), 1201(C-O), cm-1, 1H-NMR (DMSO-d6, δ): 3.62(6H, s, 2× OCH3), 4.34 (2H, s, OCH2), 4.52 (2H, s, OCH2), 6.67 (s, 2H, H-3), 6.84( 2H, d, J 8.2), 7.86 (4H, d, J 8.4), 7.93 ( 2H, d, J 8.2), 8.24( 2H, d, J 8.21,), 8.46(1H, s ,CH=N), 9.22(1H,bs, OH), 9.95 (bs, 2H, OH),14.21(1H, bs, NH) 13C-NMR (100 MHz, DMSO-d6): 56.09(2C, OCH3), 70.88(1C, CH2OCH2), 69.90(1C, CH2OCH2), 107.85(2C, CH), 116.11(2C), 125.56(1C) 129.07(2C, CH), 129.25(1C), 130.47(2C), 131.23(2C, CH), 133.09 (1C), 138.85(1C), 142.17 (1C) 151.91 (2C), 157(1C), 160.31(CH=N), 160.93 (C=N triazole),182.43(1C, C=S). HREIMs m/z =492.1461 [M˙+] (calc. for C25H24N4O5S, 492.1467).
4-((4-hydroxy-3-methoxybenzylidene)amino)-3-(4-(((4-hydroxy-3,5-dimethoxybenzyl) oxy)methyl)phenyl) -1,2,4-triazole-5-thione 7b
Recrystallized from ethanol to afford yellow precipitate. Yield 78% Mp 81-83 ˚C IR (KBr) vmax, 3568(OH phenol), 3169(NH), 3053(CHAr), 1621 (CH=N), 1605 (C=Ntriazole), 1595, 1983(C=C), 1232(C=S), 1195(C-O); cm-1. 1H NMR (DMSO-d6, δ): 3.57(6H, s, 2× OCH3), 3.89 (s, 3H, OCH3), 4.41 (2H, s, OCH2), 4.59 (2H, s, OCH2), 6.62 (s, 2H, H-3), 6.83 (d, 1H, J 7.79), 6.86( 2H, d, J 8.0), 7.26 (dd, 2H, J 8.22;1.8), 7.43 (s, 1H)) 7.91 ( 2H, d, J 8.22), 8.22( 2H, d, J 8.2), 8.59 (s, 1H, CH=N), 9.23(1H, bs, OH), 10.06 (1H bs, , OH),14.01(1H, bs, NH). 13C-NMR (100 MHz, DMSO-d6): 56.09(2C, OCH3), 56.13(1C,OCH3), 70.91(1C, CH2OCH2), 69.78(1C, CH2OCH2), 108.04(2C, CH), 110.31(1C), 116.1(1C) 116.37(2C), 124.25 (1C), 125.63(1C), 126.08(1C), 129.11(2C, CH), 129.19(1C), 130.52(2C), 131.53(2C, CH), 133.29(1C), 138.65(1C), 142.17 (1C), 148.45(1C), 151.92 (2C), 153.39(1C), 157.1(1C), 161.10(CH=N), 164.22(C=N triazole), 179.93(1C, C=S). HREIMs m/z = 522.1568 [M˙+] (calc. for C26H26N4O6S, 522.1573).
4-((4-hydroxy-3-ethoxybenzylidene)amino)-3-(4-(((4-hydroxy-3,5-dimethoxybenzyl) oxy)methyl)phenyl) -1,2,4-triazole-5-thione 7c
The crude product was recrystallized from acetonitrile to afford yellow crystals. Yield 71% Mp 104-106 ˚C. . IR (KBr) vmax, 3584(OH phenol), 3170(NH), 3037(CHAr), 2987, 2865 (CH aliphatic) 1618 (CH=N), 1607 (C=Ntriazole), 1595, 1983(C=C), 1238(C=S). 1201(C-O), cm-1. 1H-NMR (400MHz, DMSO-d6) ) 1.43 (t, 3H, J 6.9, O-CH2CH3), 3.72(6H, s, 2× OCH3), 3.92 (q, 2H, J 7.1, O-CH2), 4.39 (2H, s, OCH2), 4.74 (2H, s, OCH2), 6.54 (s, 2H, H-3), ), 6.85 (d, 2H, J 8.0, 7.24 (1H, dd, J 8.1; 1.68) 7.71 ( 2H, d, J 8.22, H-8), 8.26( 2H, d, J 8.2,), 8.59 (1H, s, CH=N), 9.16(1H, bs, OH), ), 9.78 (1H, bs, OH),14.14(1H, bs, NH), 13C-NMR (100 MHz, DMSO-d6): 14.94 (1C, O-CH2CH3), 55.21(2C, OCH3), 64.32 (1C, OCH2), 70.96(1C, CH2OCH2), 69.87(1C, CH2OCH2), 108.05(2C, CH), 111.32(1C), 117.13 (1C), 124.77 (1C), 127.51(1C), 129.39(2C, CH), 129.65(1C), 132.36(2C, CH), 134.16(1C), 138.82(1C), 142.72 (1C), 147.86 1C), 150.61 (1C) 151.89 (2C), 161.01(1C, CH=N), 163.12 (C=N triazole), 181.34(1C, C=S). HREIMs m/z = 5536.1725 [M˙+] (calc. for C27H28N4O6S, 536.1730).
4-((4-hydroxy-3,5-dimethoxybenzylidene)amino)-3-(4-(((4-hydroxy-3,5-dimethoxybenzyl) oxy)methyl)phenyl) -1,2,4-triazole-5-thione 7d
Crude product recrystallized from chloroform-ethanol to afford pale yellow precipitate. Yield 73%. Mp 136-138 °C. IR (KBr) vmax ,3605(OHphenol), 3172(NH), 3062(CHAr), 2984, 2890 (CH aliphatic) 1614 (CH=N), 1605 (C=Ntriazole), 1595, 1980(C=C), 1221(C=S), 1198(C-O), , cm-1, 1H-NMR (400MHz, DMSO-d6)3.73(6H, s, 2× OCH3), 3.85 (s, 6H, 2×OCH3) 4.42 (2H, s, OCH2), 4.56 (2H, s, OCH2), 6.59 (s, 2H, H-3), 7.24 (s, 2H) 7.71 ( 2H, d, J 8.2, H-8), 8.28( 2H, d, J 8.0,), 8,51(1H, s, CH=N), 9.08(2H, bs, OH),9.65(1H, bs, OH),13.97(1H, bs, NH)13C-NMR (100 MHz, DMSO-d6): 56.28(2C, OCH3), 56.31(2C, OCH3) 70.17(1C, CH2OCH2), 68.99(1C, CH2OCH2), 106.07 (2C, CH), 107.96(2C, CH), 129.27(2C, CH), 124.78(1C) 129.28(1C), 131.23(2C, CH), 133.43(1C), 138.66(1C), 142.41 (2C) 151.85 (4C), 160.35(1C, CH=N), 162.13 (1C, C=Ntriazole),181.22(1C, C=S). HREIMs m/z = 552.1673 [M˙+] (calc. for C27H28N4O7S, 552.1679).
4-((4-hydroxy-3,5-di-tert-butylbenzylidene)amino)-3-(4-(((4-hydroxy-3,5-dimethoxybenzyl) oxy)methyl)phenyl) -1,2,4-triazole-5-thione 7e
The crude products recrystallized from Chloroform afforded off white precipitate. Yield 60%, mp 158-160, IR (KBr) vmax, 3599.7 (OHphenol), 3177(NH), 3068 (CHAr), 2990-2885 (CHalph), 1622(CH=N), 1608(C=Ntriazole), 1595-1589 (C=C) and 1262(C=S). 1H NMR, δ 3333334.65 (2H, s, OCH2), 5.62 (1H, bs, OH), 6.64 (2H, s, H-3), 7.62 ( 2H, d, J 8.22, H-8), 7.91(s, 2H), 8.18( 2H, d, J 8.1,), 8.60(1H,s, CH=N), 9.14(1H, bs, OH),(1H, bs, NH) 13C-NMR (100 MHz, DMSO-d6): 30.37(6C, 4(C(CH3)3), 34.46(2C, C(CH3)3)) 56.11(2C, OCH3), 71.24(1C, CH2OCH2), 69.93(1C, CH2OCH2), 107.65(2C, CH), 116.86 (1C) , 124.80 (1C), 129.07(2C, CH), 129.35(1C), 131.38(2C, CH), 133.14(1C), 138.85(1C), 142.17 (1C) 151.79 (2C), 157.15 (1C), 161.77 (1C, CH=N), 165.11 (C=N triazole),182.71(1C,C=S). HREIMs m/z = 604.2715 [M˙+] (calc. for C33H40N4O5S, 604.2719).
4-((2-hydroxy-3,5-di-tert-butylbenzylidene)amino)-3-(4-(((4-hydroxy-3,5-dimethoxybenzyl) oxy)methyl)phenyl) -1,2,4-triazole-5-thione 7f
The crude products recrystallized from Chloroform-ethanol afforded pale yellow precipitate. Yield 73 %, mp 122-124˚C, IR (KBr) vmax, 3603 (OHphenol), 3173(NH), 3068 (CHAr), 2980-2885 (CHalph), 1621(CH=N), 1604(C=Ntriazole), 1597-1589 (C=C) and 1262(C=S) 1H NMR, δ (ppm, CDCl3, 400 MHz): )1.32(9H, s, o-(t-Bu),1,45(9H, s, p-(t-Bu), 3.71(6H, s, 2× OCH3), 4.42 (2H, s, OCH2), 4.45 (2H, s, OCH2), 6.57 (s, 2H, H-3),7.47(1H,d, J 2.4) 7.52(1H, d, J 2.2)7.74 ( 2H, d, J 8.22, H-8), 8.18( 2H, d, J 8.2,),8,56(1H, s, CH=N) 9.14(1H,bs, OH),10.21 1H,bs,OH), 14.29(1H,bs,NH), 13C-NMR (100 MHz, DMSO-d6): 29.32(3C, p-(C(CH3)3), 31.52(3C, o-(C(CH3)3), 34.56(1C,o– C(CH3)3), 35.41(1C,p– C(CH3)356.31(2C, OCH3), 71.02 (1C, CH2OCH2), 72.57 (1C, CH2OCH2), 106.35(1C), 108.95(2C, CH), 120.41 (1C),128.29(1C), 128.81(2C,CH), 129.32(1C), 131.24(2C, CH), 134.13 (1C),137.61(1C), 139.55 (1C), 141.7(1C), 142.17(1C) 151.91 (2C),154(1C), 162.07 (1C, CH=N), 164.08 (C=N triazole),182.88(1C,C=S) HREIMs m/z = 604.2716 [M˙+] (calc. for C33H40N4OsS, 604.2719)
Antioxidant
DPPH assay
The assay was performed as reported by Gerhauser et al. 34. Five microliters of the sample (dissolved in ethanol) was added into 195 μL of 100 μM DPPH reagent in ethanol (96%) and mixed in a 96-well plate. The intensity of the colour was measured for 3 h at an interval of 20 min at 515 nm. Ascorbic acid and BHT were used as reference.
FRAP assay
The FRAP assay was performed according to the Benzie and Strain 35 method. The FRAP reagent was prepared by combining 300 mM acetate buffer and 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) solution in 40 mM HCl and 20 mM FeCl3·6H2O, in a ratio of 10:1:1. The FRAP reagent wasincubated at 37 °C prior to use. Ten microliters of the sample was reconstituted in the carrier (solventor ultrapure water) and mixed with 300 μL of FRAP reagent. The mixture was incubated at 37 °C for 4 min in a microplate reader. The absorbance of the complex was 593 nm. The FRAP value can be calculated using the following equation36 :
FRAP = [(0–4 min ∆A593 nm of test sample)/(0–4 min ∆A593 nm of standard)]
× [standard] (µM) × Y × 1000
Where; Y is absorbance of the spectrophotometer.
Results and Discussion
Chemistry
The 4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzoic acid 4 and their corresponded acid hydrazide 5 were synthesized from according to the procedure described by K. F. Ali 33 as depicted in Scheme 1
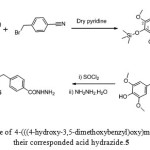 |
Scheme 1: Synthetic route of4-(((4-hydroxy-3,5-dimethoxybenzyl)oxy)methyl)benzoic acid 4 and theircorresponded acid hydrazide.5 |
The 4-amino-3-(4-(((4-hydroxy-3,5dimethoxybenzyl)oxy)methyl)phenyl)-1,2,4-triazole-5-thione. 6 were synthesized from two different methods. The first one, from melt reaction with Thiocarbonyldihydrazide for tree hour at 160-165 ˚C to obtained 51% yield. The second methods, was from converted the corresponded acid hydrazide 5 to their corresponded potassium hydrazinecarbodithioate salt as the first step. The next step was reacted this salt with hydrazine hydrate under reflux for 7h. this method exhibited yield higher than method one. The compound 7a-7f were synthesized from reaction compound 6 with hydroxyl substituted benzaldehyde in the presence of glacial acetic acid. As depicted in Scheme 2
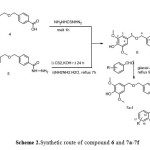 |
Scheme 2: Synthetic route of compound 6 and 7a-7f |
All synthesized compounds were characterized by IR, 1H-NMR, 13C-NMR spectrum beside the EIMS and HRMIS. The IR spectrum of compound 4 displayed rise a new band of C=O at 1676 cm-1 and disappeared the band of C≡N at 2243 cm-1.compound 5 showed shifting at carbonyl group which is appeared at 1661 cm-1 of acid to hydrazide as well new bands of NH and NH2 were appeared at 3308-3218 cm-1. The IR spectrum of compound 6 exhibited disappeared of carbonyl group and new interesting bands were appeared at 1628 cm-1 corresponded the C=N group of the triazole ring as and at 1245 cm-1 of C=S beside the value of NH and NH2 bands were shifted to 3410,3330 and 3170 cm-1 respectively. The spectra of 7a-7f displayed disappeared the band of NH2 and a new interesting band for the Schiff base was appeared at 1623-1614 cm-1 besides the C=N of the triazole ring which appeared at 1608-1604 cm-1. The 1H-NMR spectrum of compound 4 showed disappearing of tri methylsilyl group at 0.17 and appears of the broad singlet of OH for the converting the nitrile group to carboxylic acid. The 1H-NMR spectrum of compound 5 showed the disappearing hydroxyl group of the carboxylic as well a new two board singlet of NH2 and NH were appeared at 4.63 δ and 8.86 δ respectively. The 1H-NMR of compound 6 displayed all expected peaks in triazole structure. The 1H-NMR spectra of compounds 7a-7f showed the appearance of new peaks represented the Schiff base (imin group) and the expected proton of the substituted hydroxybenzylidene (see the experimental suction) also appeared in their expected rang. The 13C-NMR spectrum of compound 4 exhibited two carbons of CH2OCH2 at 3.87 & 433 δ, beside disappearing the carbon of C≡N and raised a new carbon of C=O. That enhances the evidence of successful convert compound 3 to 4 besides the spectra of IR and 1H-NMR. The 13C-NMR showed change in value of carbonyl of compound 5 due to convert the acid to acid hydrazide. The spectrum of compound 6 showed two new carbons first on the C=N at 149.54 δ of triazole ring and the second one at 166.61 δ represented the C=S. The 13C spectra of compounds 7a-7f were showed an interested peak of CH=N beside the carbons of the hydroxybenzylidene. The substituent group for 7b-7f were appeared in the expected range. The EIMS spectra showed the molecular ion M•+ for all compounds and the base peak (100%) as well the HREIMs value was confirmed the accurate mass and the molecular formula .as depicted in Table 1.
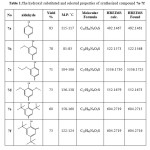 |
Table 1: The hydroxyl substituted and selected properties of synthesized compound 7a-7f Click here to View table |
Antioxidant Activity
The synthesized compound 6 and7a-7f were showed high antioxidant ability in both assays(DPPH and FRAP). Compound 6 showed antioxidant ability higher than 7a-7b in both assays and that could attributed to exhibited a pro-oxidative effect37. Even though compounds 7a-7f exhibited significant antioxidant ability. The type of substituted at hydroxybenzylidene play an important role to enhance the antioxidant ability. Compound 7e exhibited higher antioxidant ability (slightly less than ascorbic acid) in both assays (DPPH and FRAP) as shown in Figure 1and Figure 2.
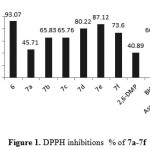 |
Figure 1: DPPH inhibitions % of 7a-7f |
Compound 7d showed free radical scavenging ability more than compound 7f and that could attributed to the t-Bu group at position para reduce the antioxidant ability38, the compound 7b and 7c exhibited nearly same antioxidant ability and that in agreement with most literatures. Finally compound 7a without any substituted group around the hydroxyl group of phenol show the less antioxidant ability.
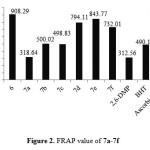 |
Figure 2: FRAP value of 7a-7f |
Conclusions
A series of newly Schiff base compounds from 4-amino-1,3,4-trizole incorporating hindered phenol moieties were successfully synthesized and characterized. All of the newly compounds were tested for antioxidant activity by DPPH and FRAP assays. The antioxidant ability of these compound increases with increasing the hindered phenol.
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
Acknowledgements
The author would like to thank the University of Malaya for running the NMR and to NUS, (Singapore) for running the EIMS and HREIMS We are also grateful to Dr. Raied Mustafa Shakir. for his contributions and Dr Mohammed Farouq Halabi, for his great help with screening,. Also, we would like to thank the university of Baghdad for supporting this study and provide the grant for this study.
References
- E. Shacter, Drug Metabolism Reviews 2000, 32, 307-326.
CrossRef - R. Chatterjee, U. Bandyopadhyay, A. Mazumdar, R. K. Banerjee, Biochem Pharmacol. 1996, 52, 1169-1175.
CrossRef - P. Sangeetha, U. N. Das, R. Koratkar, P. Suryaprabha, Free Radical Biology and Medicine 1990, 8, 15-19.
CrossRef - S. Kar, Subbaram, S., Carrico, P M., Melendez, J A., Respir Physiol Neurobiol. 2010, 174, 299-306.
CrossRef - Z. Khalil, B. Khodr, Free Radic Biol Med. 2001, 31, 430-439.
CrossRef - M. D. Mullican, M. W. Wilson, D. T. Connor, C. R. Kostlan, D. J. Schrier, R. D. Dyer, J. Med. Chem 1993, 36, 1090-1099.
CrossRef - X. Ye, W. Zhou, Y. Li, Y. Sun, Y. Zhang, H. Ji, Y. Lai, Cancer Chemotherapy and Pharmacology 2010, 66, 277-285 10.1007/s00280-009-1161-z.
CrossRef - Mohammed Farouq Halabi, Raied Mustafa Shakir, Daleya Abdulaziz Bardi, Nahla Saeed Al-Wajeeh, Abdulwali Ablat, Pouya Hassandarvish, Maryam Hajrezaie, Anwar Norazit, Mahmood Ameen Abdulla, Plos one 2014, 9, e95908.
CrossRef - C.-Y. H. A. G.-C. YEN, J. Agric. Food Chem. 2002, 50, 2993-2997.
CrossRef - R. M. Shakir, A. Ariffin, M. Abdulla, molecules 2014, 19, 3436-3449.
- J.-M. Jeong , S.-K. Kang, I.-H. Lee, J.-Y. Lee, H. Jung, C.-H. Choi, J Pharm Pharmaceut Sci 2007, 10, 537-546.
CrossRef - R. Edge, D. J. McGarvey, T. G. Truscott, J Photoch Photobio B 1997, 41, 189-200.
CrossRef - Bajroliya S, Kalwania G. S, Choudhary S, Chomal S, Oriental Journal of Chemistry 2014, 30, 1601-1608.
- L. B. Allen, K. H. Boswell, T. A. Khwaja, R. B. Meyer, R. W. Sidwell, J. T. Witkowski, L. F. Christensen, R. K. Robins, J Med Chem. 1978, 21, 742-746 10.1021/jm00206a005.
CrossRef - A. l. Montagu, V. Roy, J. Balzarini, R. Snoeck, G. Andrei, L. A. Agrofoglio, Eur J Med Chem. 2011, 46, 778-786.
CrossRef - J. P. Marino, P. W. Fisher, G. A. Hofmann, R. B. Kirkpatrick, C. A. Janson, R. K. Johnson, C. Ma, M. Mattern, T. D. Meek, M. D. Ryan, C. Schulz, W. W. Smith, D. G. Tew, T. A. Tomazek, D. F. Veber, W. C. Xiong, Y. Yamamoto, K. Yamashita, G. Yang, S. K. Thompson, J Med Chem. 2007, 50, 3777-3785 10.1021/jm061182w.
CrossRef - B. L. Wilkinson, L. F. Bornaghi, T. A. Houston, A. Innocenti, D. Vullo, C. T. Supuran, S.-A. Poulsen, J Med Chem. 2007, 50, 1651-1657 10.1021/jm061320h.
CrossRef - Y. Xia, Y. Liu, J. Wan, M. Wang, P. Rocchi, F. Qu, J. L. Iovanna, L. Peng, J Med Chem. 2009, 52, 6083-6096 10.1021/jm900960v.
CrossRef - A. M. Abdel-Megeed, H. M. Abdel-Rahman, G.-E. S. Alkaramany, M. A. El-Gendy, Eur J Med Chem. 2009, 44, 117-123.
CrossRef - M. De La Rosa, H. W. Kim, E. Gunic, C. Jenket, U. Boyle, Y.-h. Koh, I. Korboukh, M. Allan, W. Zhang, H. Chen, W. Xu, S. Nilar, N. Yao, R. Hamatake, S. A. Lang, Z. Hong, Z. Zhang, J.-L. Girardet, Bioorg Med Chem Lett. 2006, 16, 4444-4449.
CrossRef - M. A. Hussein, R. M. Shaker, M. A. Ameen, M. F. Mohammed, Archives of Pharmacal Research 2011, 43, 1239-1250.
CrossRef - I. Khan, S. Ali, S. Hameed, N. H. Rama, M. T. Hussain, A. Wadood, R. Uddin, Z. Ul-Haq, A. Khan, S. Ali, M. I. Choudhary, Eur J Med Chem. 2010, 45, 5200-5207.
CrossRef - X. Shan, A. O. Ibrahim, Y. Zhou, H. Zhang, J. Ma, F. Jiang, M. Hong, Inorganic Chemistry Communications 2012, 22, 149-153.
CrossRef - S. Ogiri, M. Ikeda, A. Kanazawa, T. Shiono, T. Ikeda, Polymer 1999, 40, 2145-2150.
CrossRef - H. Liu, Z.-e. Fu, K. Xu, H.-l. Cai, X. Liu, M.-C. Chen, Materials Chemistry and Physics 2012, 132, 950-956.
CrossRef - S.-H. Kim, S.-Y. Gwon, S. M. Burkinshaw, Y.-A. Son, Dyes and Pigments 2010, 87, 268-271.
CrossRef - M. Chandra, A. N. Sahay, D. S. Pandey, R. P. Tripathi, J. K. Saxena, V. J. M. Reddy, M. Carmen Puerta, P. Valerga, Journal of Organometallic Chemistry 2004, 689, 2256-2267.
CrossRef - A. Bodtke, W.-D. Pfeiffer, N. Ahrens, P. Langer, Bioorganic & Medicinal Chemistry Letters 2004, 14, 1509-1511.
CrossRef - İ. Kaya, N. Cihangiroğlu, Journal of Polymer Research 2004, 11, 37-42.
CrossRef - A. I. Khodair, P. Bertrand, Tetrahedron 1998, 54, 4859-4872.
CrossRef - Huihui Ti, Qing Li, Ruifen Zhang, Mingwei Zhang, Yuanyuan Deng, Zhencheng Wei, Jianwei Chi, Yan Zhang, Food Chemistry 2014, 159 166-174.
CrossRef - M. N. Fausta Natella, Maurizio Di Felice, and Cristina Scaccini, N. I. o. N. Free Radical Research Group, Roma, Italy, J. Agric. Food Chem. 1999, 47, 1453-1459.
- K. F. Ali, Oriental Journal of Chemistry 2015, 31, 239-247.
- C. Gerhäuser, K. Klimo, E. Heiss, I. Neumann, A. Gamal-Eldeen, J. Knauft, G.-Y. Liu, S. Sitthimonchai, N. Frank, Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2003, 523-524, 163-172 10.1016/S0027-5107(02)00332-9.
CrossRef - I. F. Benzie, J. J. Strain, Anal. Biochem. 1996, 239, 70–76 10.1006/abio.1996.0292.
CrossRef - A. Rustaiyan, Javidnia, K., Farjam, M H., Aboee-Mehrizi, F., Ezzatzadeh, E., J Med Plants Res. 2011, 5, 4251-4255.
- E. Sergedien, K. Jönsson, H. Szymusiak, B. Tyrakowska, I. M. C. M. Rietjens, N. Čenas, FEBS letters 1999, 462, 392-396.
CrossRef - R. H. Rosenwald, J. R. Hoatson, J. A. Chenicek, Industrial & Engineering Chemistry 1950, 42, 162-165 10.1021/ie50481a042.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









