Potassium fluoride/K10-montmorillonite nano structure as a Green and Reusable Catalyst under mild reaction conditions for the knovenagel Condensation
Mercedeh Ghanei1, Mohammad A. Khalilzadeh2,*and Mohammad M. Hashemi1
1Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran 2Department of Chemistry, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran Corresponding author e-mail: m.khalilzadeh@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/320175
Article Received on :
Article Accepted on :
Article Published : 19 Feb 2016
Potassium fluoride (KF)/montmorillonite (K10-MMT) nanostructure acts as an active heterogeneous base catalyst for Knovenagel condensation. Here we have described the catalytic activities of KF/K10 nanostructure in the condensation reaction of various substituted benzaldehydes with active methylene compound malononitrile under mild condition. The experimental results showed that the KF/K10 had high catalytic activity and it can be recycled without significant loss of activity. This practical and eco-friendly protocol provides a facile way to C-C bond formation under mild conditions.
KEYWORDS:Potassium fluoride; Montmorillonite; Carbon-Carbon bond; Solid base catalyst; Knovenagel condensation
Download this article as:| Copy the following to cite this article: Ghanei M, Khalilzadeh M. A, Hashemi M. M. Potassium fluoride/K10-montmorillonite nanostructure as a Green and Reusable Catalyst under mild reaction conditions for the knovenagel Condensation. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Ghanei M, Khalilzadeh M. A, Hashemi M. M. Potassium fluoride/K10-montmorillonite nanostructure as a Green and Reusable Catalyst under mild reaction conditions for the knovenagel Condensation. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14061 |
Introduction
Formation of C-C bonds is one of the significant objectives in organic synthesis, particularly in the synthesis of fine chemical products. Knoevenagel condensation is one of the well well documented reactions for forming electrophilic olefins and C–C bond between carbonyl compounds and active methylene compounds. This reaction is a practical method to achieve fine chemical intermediates and therapeutic and pharmacological products [1-3].
Owing to their importance from an industrial, pharmacological and synthetic point of view a great number of methods for the Knoevenagel condensation have been developed using various Lewis acids/bases under ultrasound and microwave irridiation, using green solvent like water or ionic liquid, and grindstone method under solvent-free condition [4-12]. However, most of the reported reagents suffer from disadvantages such as high cost reagent, tedious work-up, environmental limitations, disposal problems and so on. In order to cumbersome these problems, emphasis has been laid on the use of supported reagents. The major advantage of supported reagent is the reusability of the catalyst that makes the process inexpensive. Furthermore, it also contributes towards the area of “Green Chemistry” [13].
Solid base is a vital variety of catalysts offering excellent opportunities for replacing of reducing operating costs associated with base neutralization and product purification, diminishing corrosion and other related environmental problems, and in the meantime allowing easier separation and reusable of the catalysts. Moreover, solid bases also have the advantages of nontoxicity, eco-friendliness and easy work-up procedure [14-16].
Among the salts with potentially basic properties potassium fluoride (KF) is more general owing to its cheapness and availability. A lot of supports have been introduced for increasing the basicity of KF such as KF/ZnO, KF/Ca–Mg–Al, KF/CaO–Fe3O4, KF/celite, KF/zeolite, and KF/LDH mainly applied for organic production. Although, a majority of the abovementioned solid bases are effective in organic transformations, they suffer from some drawbacks such as low basicity, use of expensive solid supports and tedious preparation steps [17-25].
Montmorillonite (MMT) clay having two silica tetrahedral sheets layered between one alumina octahedral sheets. MMT is a clay mineral with a large specific exhibits good adsorbability, surface area, cation exchange capacity. K10-MMT is known to behave as heterogeneous catalysis in organic reactions. Consequently, we thought to employ a new type of solid acid–base catalyst, where the base is electrostatic bond onto a solid acidic support towards the desired reaction [26-28].
K10-MMT has high cation exchange capacity specifically, so use of this property by impregnation of potassium fluoride on MMT reasons a more free fluoride anion, which is able to act as an effective base [29].
With attention to above details, this research indicated that K10/KF nanostructure can be used as an suitable heterogeneous basic catalyst for solvent-free methods based on green chemistry to improve the Knoevenagel condensation reaction by condensation of phenolic compounds with methyl acetoacetate. Finally In the context of our work this catalyst is desirable alternative, owing to its non-toxicity, low-cost and eco-friendly properties.
Experimental
Materials and Methods
All starting materials and chemical solvents were purchased from Aldrich and Merck were used without further purification.
Preparation of the catalyst
The catalyst was prepared according to the literature procedure; prepared by dissolving KF (5.5 g) in distilled water (10 mL) and K10-MMT (10 g). The mixture was stirred for 1 h next, the water was removed at 60–70 °C in a rotary evaporator under reduced pressure. The resulted solid mixture was further dried at 70–80 °C in a vacuum drying oven for 30 h.
Typical procedure for the Knoevenagel condensation
In a typical reaction, substituted aromatic aldehyde (1 mmol), malononitrile (1.01 mmol), and 0.1 g of catalyst (KF/K10) were placed in a glass flask equipped with a magnetic stirrer at 100 °C for 10-25 min. Then, the reaction was allowed to cool to room temperature after completion of the reaction (monitored by TLC). Then a solid precipitate was separated out, filtered and washed with water (3 x 10 mL) followed by ethanol (3 x 10 mL), and dried under vacuum. In this case approximate temperature was obtained by using sealed capillaries containing compounds of know melting point within the reaction container.
Results and Discussion
Catalytic applications of KF/K10 for Knoevenagel condensation
The catalytic activity of KF/K10 nanostructure was used as an appropriate heterogeneous basic catalyst for solvent-free protocol Knoevenagel condensation by condensation of benzaldehyde with malononitrile.
To optimize the reaction conditions, benzaldehyde and malononitrile were selected as the model substrates to examine the effects of different solvents and molar ratios of the catalyst at different temperatures. The effect of different solvents on the course of reaction was studied (Table 1). From the results given in Table 1, it was found that among various solvents tried, ethanol was found to give optimum results in term of reaction time and yield. Consequently, the optimum conditions for the reaction are: benzladehyde (1 mmol), malononitrile (1.01 mmol) and 0.16 g of KF/K10 nanostructure, 60 °C was found to be the optimum reaction temperature as at low temperature, reaction time was longer.
Table 1: The effect of different solvents on the course of reaction
|
Yield % |
Time (min) |
Solvent |
|
|
45 |
80 |
Acetonitrile |
1 |
|
60 |
90 |
Water |
2 |
|
94 |
10 |
Ethanol |
3 |
|
35 |
180 |
Chloroform |
4 |
|
80 |
30 |
Solvent-free |
5 |
In order to generalize the scope of reaction, a variety of aldehydes was subjected for reaction with malononitrile under the mild optimized reaction conditions, and the results are presented in Table 2. The spectral data and melting points are in good agreement with those reported in literature. The reactions went on well to afford products in good to high yields and short times.
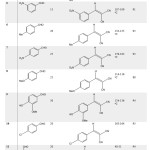 |
Table 2: Knoevenagel reaction catalyzed by KF/K10
|
It was concluded that the reuse of a heterogeneous system and the lifetime of the catalyst are highly preferable in terms of green chemistry. This research used the recycled catalytic five times without any reduction in the chemical reaction. The efficiency of the catalytic activity after five times recycling reached to 83% down from 94% as illustrated in Table 3.
Table 3: Recycling of KF/K10 nanostructure in Knoevenagel reaction by condensation reaction of benzaldehyde and malononitrile
| Cycle | Time (min) | Yield (%) |
| 1 | 12 | 94 |
| 2 | 12 | 92 |
| 3 | 15 | 90 |
| 4 | 20 | 86 |
| 5 | 20 | 83 |
Conclusions
In this research, we supported KF on the MMT. The research found that KF/K10 is a proper nanocatalyst with a high stability for Knoevenagel condensation. The advantages of the present method are simplicity of work-up, high yields, short reaction times and recyclability of the catalyst.
References
- Knoevenagel E., Berichte der Deutschen Chemischen Gesellschaft.1894,2, 2345-2346.
CrossRef - Knoevenagel E., Chemische Berichte.1898,31, 2585-2595.
CrossRef - Jones G., Organic Reactions.1967,15, 204-599.
- Gill C., Pandhare G., Raut R., Gore V. Gholap S., Bull. Cat. Soc. India.2008,7,153-157.
- Mogilaiah K., Reddy CS., Synt. Comm.2003,33,3131-3134.
CrossRef - Mallouk S, Bougrin K, Laghzizil, Benhida R., Molecules.2010,15,813-823.
CrossRef - Bhuiyan MMH, Hossain MI, Ashraful M., Mahmud MM., Chemistry Journal.2012,2,30-36.
CrossRef - Li G., Xiao J., Zhang W., Green Chem.2012,14,2234-2238.
CrossRef - Bhat S. I., Choudhury A. R., Trivedi D. R., RSC Adv.2012,2,10556-10558.
- Zhang Y., Dou Q., Dai L., Wang X., Chen, Y., RSC Adv.2012,2,8979-8982.
- Khan FA, Dash FJ, Satapathy R., Upadhyay SK Tetr. Let.2004,45,3055-3058.
CrossRef - Verdia P, Santamarta F & Tojo E Molecules. 2011,16, 4379-4388.
CrossRef - Anastas PT & Warner JC Green Chemistry: Theory and Practice, Oxford University Press, New York, USA.2000
- Ono Y., J. Cat al.2003, 216, 406–415.
CrossRef - Hattori H., Appl. Catal.,2001,222, 247–259.
CrossRef - Weitkamp J., Hunger M., Rymsa U., Micro. Meso. Mater.2001, 48, 255–270.
CrossRef - Xie W. L., X. Huang M., Catal. Lett.2006,107, 53–59.
CrossRef - Gao L. J., Teng G. Y., Lv, J. H., Xiao, G. M., Energy Fuels.2010,24, 646–651.
CrossRef - Hu S., Guan Y., Wang Y., Han H., Appl. Energy.2011,88,2685–2690.
CrossRef - Ando T., Yamawaki J., Chem. Lett.1979,45–46.
CrossRef - Zhu J. H., Chun Y., Qin Y., Xu Q.-H., Micro. Meso. Mater.1998,24, 19–28.
- Asseid, F. M. Duke C. V. A., Miller J. M., Can. J. Chem.1990,68,1420–1424.
CrossRef - Ichihara J., Matsuo T., Harafusa T., Ando T., J. Chem. Soc., Chem. Commun.1986,793–794.
CrossRef - Zahouily M., B. Bahlaouane, M. Aadil, A. Rayadh and S. Sebti, Org. Process Res. Dev., 2004,8,275–278.
CrossRef - Gao, L., Teng G., Xiao G., R. Wei, Biomass Bioenergy,2010,34,1283–1288.
CrossRef - Laszlo P., Science.1987, 235,1473-1479.
CrossRef - Vaccari A., Catal. Today.1998,41, 53-56.
CrossRef - Motokura K., Tada M., Iwasawa Y., J. Am. Chem. Soc.2009,131,7944-7947.
CrossRef - (a) Miller, J. M. So, K.-H., Clark, J. H.J. Chem. Soc. Chem. Commun.1978,466–467.
(b) Clark, J. H. J. Chem. Soc. Chem. Commun.1978,789–791.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









