Galactose oxidase nanoaggregates: Preparation and characterization
Mamta Sharma1 and Minakshi Sharma*
Department of Zoology, Mahrashi Dayanand University, Rohtak-124001, Haryana,India Corresponding author email: sminakshi.2007@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/320158
Article Received on :
Article Accepted on :
Article Published : 22 Feb 2016
Galctose oxidase nanoaggregates have been prepared by chemical desolvation method involving the crosslinkng agent glutaraldehyde. These enzyme nanoagregates have been characterized by transmission electron microscopy(TEM), UV visible spectroscopy, Fourier transform infrared spectroscopy (FTIR). TEM reveals the globular spherical nanostructured form upto the range of 20nm. UV visible spectroscopy of galactose oxidase nanoaggregates shows maximum absorption peak at 237nm. FTIR spectra obtained a C-N bending at 1634cm-1 due to glutaraldehyde crosslinking. NP aggregates were more stable, active and had a higher shelf life than that of free enzyme and reused many times.
KEYWORDS:Nanoaggregates; Spectroscopy; structure; Galactose oxidase; microscopy
Download this article as:| Copy the following to cite this article: Sharma M, Sharma M. Galactose oxidase nanoaggregates: Preparation and characterization. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Sharma M, Sharma M. Galactose oxidase nanoaggregates: Preparation and characterization. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14142 |
Introduction
The best excellency and biocompatibility of various nanoparticles with biomolecules possess unique structural, magnetic, optical, catalytic properties and also enhance the conductivity of biosensor in terms of sensitivity[1]. Enzyme molecules were precipitated as insoluble aggregates with their native confirmation and cross linked by bifunctional cross linkers [2,3].Cross linking of enzyme via glutaraldehyde with reactive NH2 groups on protein surface was developed more than 40 year ago. Cross-linked enzymes are produced by mixing both solution of enzyme and glutaraldehyde [4,5]. Enzyme nanoaggregates could be reused over again and again and also have a longer shelflife and stability over denaturation by heat and organic solvents and problems related to enzyme leaching is not associated with these aggregates [6,7].Cross linked enzyme NP aggregates could be immobilized on specific support using high proportion of active enzyme. Enzyme nanoaggregates are supramolecular structure held by non covalent interactions[8].These enzyme nanoaggregates play important role as industrial and biomedical catalyst. Variations have been found in terms of pH, temperature in biosensors which results in leaching and denaturation of enzyme [9]. Because of these problems, the enzyme nanaparticles based biosensor achieve superior design as it is not time consuming and non-laborious process [10].
Although various aggregates of enzyme NPs were formed by this method like of glucose oxidase, trypsin, lipase, laccase, phytase, penicillin amylase, tyrosinase, cholesterol oxidase, horseradish peroxidase and they have wide applications like in pharmaceutical, food, detergent ,bioreactore, biofuels, biomedical devices[8-10]. The galactose oxidase nanoaggregates are more stable to denaturation by solvents, heat and proteolysis in comparision to soluble form of enzyme[11].So, the present work describes the preparation of galactose oxidase nanoaggregates by simple method and its characterization.
Experimental
Materials and methods
Reagents- Galactose oxidase, horseradish peroxidase and Glutarldehyde (25%) were from sigma-aldrich, USA. Dextrose, cysteamine dihydrochloride, ethanol, methanol were from Himedia laboratory chemicals, India. All other reagents were of analytical reagent (AR) grade. Double distilled water (DW) was used in all experiments.
Instruments-UV-visible spectroscopy, Fourier transform infrared (FTIR) spectrophotometer ( Bruker) was carried out at Department of Zoology, M.D University, Rohtak , Spectronic 20 (Thermo, USA) for measuring A425nm. Transmission electron microscopy (TEM) carried out at PU, Chandigarh.
Assay for Free Galactose oxidase (GalOx)
GalOx assay was carried out as per Aries et.al.,1992 [14] with modifications. It was carried out in 15ml test tubes wrapped with black paper. Assay mixture contained 1.8ml of 0.1M potassium phosphate buffer(PB, pH 6.0),0.1ml enzyme (1mg/ml) and 0.1ml galactose (360mg/ml) as substrate. After incubating the reaction mixture at 37oC for 2 min, 1 ml of colour reagent was added and kept at room temperature for 15min to develop the color. A425 nm was read in spectronic -20 and H2O2 generated was determined from standard curve between H2O2 versus A425. The color reagent consisted of 50mg of 4-aminophenazone, 100mg phenol(solid),and 1mg horseradish peroxidase in 100ml of 0.4MPB, pH 7.0 and stored in amber colored bottle at 4C.
Preparation of Galactose oxidase Nanoparticles (GalOxNP) Aggregates.
The GalOx-NP aggregate havebeen prepared as per Liu et al., 2005[1] by desolvation method with modification . In this method first of all 6ml of absolute ethanol was added to 3ml of enzyme solution (1mg/ml) at a droping rate of 0.1-0.2 ml/min under constant stirring at 500 rpm resulting into aggregation of enzyme/protein molecules into smaller particles/nanoparticles. After then,1.8 ml of 2.5% glutaraldehyde was added to these NPs suspensions with continous stirring at 500rpm at 4oC for 24hr, thus ensuring the crosslinking of enzyme nanoarticles. Amino group were introduced on GalOxNPs by addition of 0.12g of cysteamine dihydrochloride with 5-6hr of continous stirring. Synthesized GalOxNP were then separated from free enzyme solution by centrifugation at 12000rpm at 4oC for 10 min followed by dispersion in 0.1M PB (pH 6.0) with sonication for 5min. It is assumed that –NH2 group of cysteamine reacts with –CHO group of glutaraldehyde cross linked galactose nanoparticles to form Schiff base. These nanoparticles aggregates further characterized by UV spectroscopic, TEM and FTIR spectroscopic studies. GalOxNPs were stored at 4oC, for further use. The scheme is shown below.
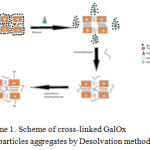 |
Scheme 1: Scheme of cross-linked GalOx nanoparticles aggregates by Desolvation method. Click here to View scheme |
 |
Figure 1: Synthesized Galactose oxidase nanoparticles. Click here to View figure |
Characterization of Galactose oxidase nanoparticles
The aggregated galactose oxidase nanoparticles were studied by Transmission electron microscopy (TEM) at Punjab University, Chandigarh, FTIR spectroscopy (BRUKER) ,UV visible spectroscopy(Spectronic-20) at Maharshi Dayanand University, Rohtak. Samples are prepared by dispersing drop of colloid on copper grid, covered with the carbon film and the solvent is evaporated. UV visible spectroscopy of both free Galactose oxidase and GalOxNPs were measured in spectronic-20 at different wavelengths.
Results and Discussion
Transmission electron microscopy
TEM image of prepared GalOxNPs showed their spherical shape with diameter in the range of upto 20nm (Fig.2). GalOxNPs have a hollow spherical structures on the electron microscopic images. The TEM image showed GalOxNPs aggregates with an average diameter of 100nm
Fourier Transform infrared spectroscopy (FTIR)
FTIR spectra of free Galactose oxidase showed the vibration bands at 1090 and 1405cm-1 confirm the presence of amide I and amide II infrared bands. These bands are used in conformational changes monitoring. The amide I band is due to C=O stretching of peptide linkages while amide II band is due to combination of N-H bending and C-N stretching of peptide linkages.whereas the FTIR spectra of GalOxNPs aggregates obtained a vibration band at 1634cm-1 due to C=N stretching formed after glutaraldehyde reaction,confirming the formation of GalOxNPs aggregates also with the vibration bands of amideI and amideII having its enzymatic activity. There is also a broadening of vibration band at 2000-2500cm-1 in case of GalOxNPs confirming the presence of more free amine(NH2) groups, introduced by cysteamine dihydrochloride (Fig.3).
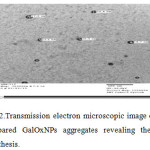 |
Figure 2: Transmission electron microscopic image of prepared GalOxNPs aggregatesrevealing their synthesis. Click here to View figure |
UV Visible spectroscopy
UV visible spectra of free galactose oxidase and galactose oxidase nanoparticles were studied separately. Free galactose oxidase showed a characterstic absorption peak at 220nm(Fig.4A) and Galactose oxidase nanoparticles aggregates showed a absorption peak at 237nm(Fig.4B).This shift in absorption maxima is the indicative of structural conformation of enzyme after nanoparticles synthesis. The peak observed in enzyme nanoparticles synthesis is due to the absorption by free amino acid groups whereas the peak shown in case of free enzyme is due to absorption by peptide bonds,which indicates the synthesized galactose oxidase nanoparticles.
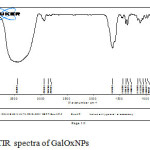 |
Figure 3: FTIR spectra of GalOxNPs Click here to View figure |
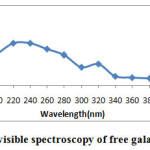 |
Figure 4A: UV visible spectroscopy of free galactose oxidase Click here to View figure |
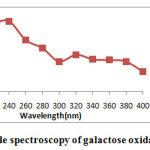 |
Figure 4B: UV visible spectroscopy of galactose oxidase nanoparticles Click here to View figure |
Conclusion
Galactose oxidase nanoparticles can improve the prospects of clinical applications of enzyme drugs
Use of these synthesized enzyme nanaoparticles can offer advantage in genetic engeeneering and clinical trials for diagnose the disorders related to galactose metabolism. These enzyme nanoaggregates also have various application in context of industrial and biomedical biocatalyst. They play a major role in biosensing platform to increase shelflife and operational stability. Direct immobilization of enzyme nanoparticles with proper choice of functionalization seems to be a promising strategy for biosensor development with high sensitivity, affinity, response time.
Author Contributions
Minakshi Sharma Conceived the Plan, Mamta Sharma performed the experiment and data analysis. Authors have no competing financial interests.
References
- Liu G, Lin Y, Ostatna V, Wang J. Enzyme nanoparticles based electronic biosensor. Chem Commun, 2005, 27, 3481–348.
CrossRef
- Kundu N, Yadav S, Pundir CS, Preparation and characterization of glucose oxidase nanoparticles and their application in dissolve oxygen metric determination of serum glucose. J Nanosci. Nanotechnol, 2012, 12,1–7.
- Boller T, Meier C, Menzler S, EUPERGIT oxirane acrylic beads: how to make enzymes fit for biocatalysis. Org Process Res Dev,2002, 6,509–519.
CrossRef - Cao L, Van-Rantwijk F, Sheldon RA ,Cross-linked enzymes aggregates: a simple and effective method for the immobilization of penicillin acylase. Org Lett ,2000, 2,1361–1364.
CrossRef - Cao,L.,Van- Langen,L.M., Sheldon,R.A. Curr.Opin. Biotechnol.2003,14,387.
CrossRef - Khare,S.K.,GuptaR,M.N.Biotechnol Bioengg,1999.
- Cao L, Van-Langen L, Sheldon RA, Immobilized enzymes: carrier bound or carrier free. Curr Opin Biotechnol ,2003,14, 387–394.
CrossRef - Kundu N, Yadav S, Pundir CS , Preparation and characterization of glucose oxidase nanoparticles and their application in dissolve oxygen metric determination of serumglucose.J.Nanosci Nanotechnol,2012, 12,1–7.
- SheldonR.A.Cross-linkedenzyme aggregates as industrial biocatalysts. Org Process Res Dev ,2011,5:213–223.
CrossRef - Ayhan F, Dogae I.Y and Ayhan H. Hacellepe,J.Biol. Chem.,2011, 39,241.
- Van-Langon LM, Selassa RP, Van-Rantwijk F, Sheldon RA, Cross-linked aggregates of R-oxynitrilase: a stable, recyclable biocatalyst for enantioselective hydrocyanation.Org Lett ,2005,7,327–329.
CrossRef - Schoevaart R, Wolbers MW, Golubovic M, Ottens M, Kieboom APG, Van-Rantwijk F, Preparation, optimization and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol Bioeng,2004, 87,754–762
CrossRef - Shah S, Sharma A, Gupta MN ,Preparation of crosslinked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal Biochem ,2006,351,207–213.
CrossRef - Arias E.B., Cancho J.C., Gonzalez D.L., Serrano J.M., Use of an enzyme assay in student classes, Biochem. Educ.,1992,20, 234–235.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









