Fabrication of nanosized Ag colloids by hydrazine hydrate
Behrad Mosavar Rahmani and Hamid Reza Ghorbani*
Department of Chemical Engineering, Central Tehran Branch, Islamic Azad University, Tehran, Iran
Corresponding author: Email: hamidghorbani6@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320152
Article Received on :
Article Accepted on :
Article Published : 24 Feb 2016
In this work, we develop a simple method to synthesize nanosized Ag colloids with the addition of hydrazine hydrate. UV–vis absorption spectra, dynamic light scattering (DLS) and transmission electron microscopy (TEM) have been used to trace the growth process and elucidate the structure of the silver nanoparticles. A solution in its UV–Vis absorption spectrum showed surface plasmon resonance absorption bands about 421 nm. We obtain silver colloids of average size 92 nm with a standard deviation of 30 nm after separation and washing procedures.
KEYWORDS:chemical synthesis; Ag colloids; hydrazine hydrate
Download this article as:| Copy the following to cite this article: Rahmani B. M, Ghorbani H. R. Fabrication of nanosized Ag colloids by hydrazine hydrate. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Rahmani B. M, Ghorbani H.R. Fabrication of nanosized Ag colloids by hydrazine hydrate. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14233 |
Introduction
Due to its excellent electrical, thermal, optical and/or catalytic properties, nanosized silvercolloids have received a great deal of attention in recent years for various potential applications, such as conductors, catalysts, chemical sensors, etc. it is important to synthesize colloidal silver dispersions with small sizes and narrow distribution. Silvert et al. (1997) obtained fine sized Ag particles whose size distribution was between 15 and 36 nm by polyol reduction. Despite these efforts, it remains difficult to prepare silver particles of less than 20 nm and with a narrow distribution from an aqueous solution. For the polyol process, the reaction temperature must consistently remain at 120 8C or higher, which adds to the cost of production. Much finer particles (less than 10 nm) can be fabricated using micelles and the micro emulsion method, but this also requires the use of organic solvents [1]. Further, the initial concentration of silver precursor in this method is still limited to less than 0.05 M for size control purposes [2, 3, 4]. Citrate route developed by Turkevich et al. is an excellent method for the preparation of aqueous colloidal solution of silver and gold. Direct large-scale synthesis can be done by various chemical methods but we opine that the use of additional mild reducing agent may offer better particle morphology via an intermediate ionic state [5]. Thus silver powder of modified surfaces with controlled morphology can be prepared by several methods including the reduction of silver nitrate by reducing agents [6, 7].
We herein describe an easy synthetic route for silver nanoparticles by hydrazine hydrate as reducing agent. The nanoparticles were characterized by various techniques.
Materials and methods
All the chemicals and reagents used were of analytical grade. An aqueous solution of hydrazine hydrate was added to an aqueous solution of silver nitrate (0.1 M). The drop-wise addition under continuous stirring at room temperature afforded a precipitate. After the complete addition of hydrazine hydrate, stirring was continued for an additional 5 min. The reduction of the Ag+ ions by hydrazine hydrate in the solutions was monitored by sampling the aqueous component (2 ml) and measuring the UV–Vis spectrum of solutions. Particle-size distributions of the samples were also obtained using dynamic light scattering (DLS). Furthermore, the silver nanoparticles were characterized by transmission electron microscopy (TEM).
Results and Discussions
It is well known that silver can be reduced from Ag+ to Ag0 by various reducing agents same hydrazine. The absorption band at about 421 nm is known to be due to surface plasmon resonance in nano-silver solutions. In fact, the energy of absorption would depend on the degree of plasmon resonance i.e. it may shift either side of this value depending on the ratio of silver ions and zero-valent silver. The UV–Visible spectrum (Fig. 1) of the solution showed a well-defined surface plasmon resonance at ∼421 nm. The technique outlined above has proven to be very useful for analyzing nanoparticles [8].
Dynamic light scattering is a used method for the determination of nanoparticle size. The silver particles’ size histograms show that the nanoparticles size is 92± 30 nm (Fig. 2). TEM micrograph of silver colloidal particles is shown in Fig. 3. It can be noticed that the graphs show that the particles are spherical in shape.
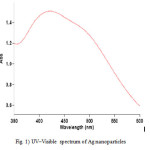 |
Figure 1: UV–Visible spectrum of Ag nanoparticles |
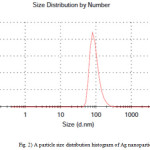 |
Figure 2: A particle size distribution histogram of Ag nanoparticles |
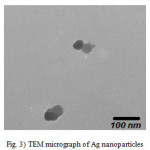 |
Figure 3: TEM micrograph of Ag nanoparticles |
Conclusions
Colloidal silver nanoparticles were synthesized by hydrazine hydrate. The size of the silver nanoparticles was 92± 30 nm. UV–Vis spectroscopy confirmed silver nanoparticles. Also, nanoparticles were characterized by DLS and TEM. From a technological point of view, these obtained silver nanoparticles have potential applications in the various fields and this simple procedure has several advantages such as cost effectiveness and scale commercial production.
References
- Silvert, P. Y.; Urbina, R. H.; Elhsissen, K. T. J. Mater. Chem., 1997, 7, 293.
CrossRef - Egorova, E. M.; Revina, A. A. Colloids Surf. A: Physicochem. Eng. Asp., 2006,168, 87.
CrossRef - Xie, Y.; R. Ye; Liu, H. Colloids Surf. A: Physicochem. Eng. Asp., 2006, 279, 175.
CrossRef - Xue, F.; Liu, Z.; Su, Yi.; Varahramyan, K. Microelectron. Eng., 2006, 83, 298.
CrossRef - Ghorbani, H.R. Orient J chem., 2015, 31(1), 303.
- Maleknia, L.,; Rashidi, A. S. Orient J chem., 2015, 31(1), 257.
- Joerger, R.; Klaus, T.; Granqvist, C.G. Adv Mater, 2000, 12, 407.
CrossRef - Ghorbani, H.R.; Soltani, S. Orient J chem., 2015, 31(1), 303.

This work is licensed under a Creative Commons Attribution 4.0 International License.









