Chitosan and reinforced Chitosan films for the removal of Cr(VI) heavy metal from synthetic aqueous solution
Sibi Srinivasan1*, Pragathiswaran Chelliah2, Venkatesan Srinivasan1, Anthuvan Babu Stantley1, and Kullagounder Subramani3
1Department of Chemistry, Adhiyamaan College of Engineering, Hosur, Tamilnadu, India.635109 2Department of Chemistry, Government Arts College, Tiruchi, Tamilnadu, India. 3Department of Chemistry, Islamiah College, Vaniyambadi, Tamilnadu, India. Corresponding Author Email: sibi_chakravar@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/320176
Article Received on :
Article Accepted on :
Article Published : 05 Feb 2016
Cr(VI) is removed efficiently from aqueous solution using Chitosan made films as the adsorbent. The efficiency of free Chitosan films is compared with the Chitosan-Silica and Chitosan-Carbon, which are reinforced with biogenic Silica and Carbon obtained from Panicum miliare husk ashes, respectively. All the films were prepared by simple ageing method and swelling index was determined for all the three adsorbents independently. Adsorption studies of Cr(VI) heavy metal were carried out by varying pH, temperature, initial concentration of the adsorbate and quantity of adsorbent. On the otherhand, Cr(VI) was removed from the solution by Chitosan-Silica film better than free Chitosan and Chitosan-Carbon films. The optimum pH was found to be 3.0 and optimum temperature was 30°C. Thermodynamic, equilibrium and kinetic studies were carried out for all the three adsorbents, independently. Langmuir adsorption isotherm and Pseudo First Order kinetics were followed for the adsorption process. Concentration of metal ions was determined using a spectrophotometer. Oxidation states of the adsorbed Cr were determined by ESR. It was found that Chitosan-Silica film reduced Cr(VI) to Cr(III) almost completely in the aqueous solution.
KEYWORDS:Chitosan; removed efficiently; aqueous solution; independently
Download this article as:| Copy the following to cite this article: Srinivasan S, Chelliah P, Srinivasan V, Stantley A. B, Subramani K. Chitosan and reinforced Chitosan films for the removal of Cr(VI) heavy metal from synthetic aqueous solution. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Srinivasan S, Chelliah P, Srinivasan V, Stantley A. B, Subramani K. Chitosan and reinforced Chitosan films for the removal of Cr(VI) heavy metal from synthetic aqueous solution. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=13867 |
Introduction
Hexavalent Cr(VI), which is highly hazardous [1, 2] exists as the chief constituent in the effluents of leather tanning and other industries [3]. Cr(VI) is known to cause various health effects. When it is a compound in leather products, it can cause hypersensitive responses, such as skin rash, itching etc. After breathing it in Cr(VI) can often cause nose irritations and nosebleeds. Other health problems that are caused by Cr(VI) are skin rashes, Upset stomachs and ulcers, respiratory problems, weakened immune systems, Kidney and liver damage, alteration of genetic material, Lung cancer and even death. Thus, there is an urgent demand for the growth of a wastewater treatment method, which would leave the low concentrations of Cr to be pulled immediately from the effluents and further enriched to a higher level for reuse in electroplating and other manufactures. Adsorption of Cr from the aqueous solution can be performed using adsorbents like clay [7], activated carbons, bio-materials, bio-wastes, hydrogels, etc. Even micro-organisms are found effective in doing this removal of this specific heavy metal [8]. Hydrogels are capable of absorbing large quantities of water and swell accordingly. The harmless and cheap nature of Chitin has allowed to use them widely in cosmetics, feed additives, packing and so on. Nowadays, several technologies using microorganisms, Chitosan have been tested as ways of removing heavy metals from the environment [1-6]. The nitrogen from the amino group of Chitosan acts as an electron donor is believed to be the prime responsible site for the adsorption of metal ions [9]. In this study, an analogy is developed to remove the Cr(VI) heavy metal in real polluted solution and the synthetic solution. The adsorption efficiency of Chitosan was studied by reinforcing with the Carbon rich and SiO2 rich ashes, independently. The chemical, physical and optical changes in the films, resulting from these combinations were already reported by us [10].
Material and Methods
Highly viscous Chitosan (99.9% pure) was purchased from Sigma-Aldrich, Bangalore, India. Chitosan powder is mixed with 5% acetic acid.
Preparation and reinforcement of film
 |
Figure1: Photo images of a)freeChitosan film b) Chitosan-Carbon composite film c) Chitosan-Silica composite film Click here to View figure |
Husks of Panicum miliare were obtained from the local farmers and were made into ashes viz., Carbon-ash and Silica-ash, respectively the Carbon rich ash and Silica rich ash. The method of preparation of ash followed as in the literature [11]. These ashes were introduced into the network of Chitosan and the resulting films with a diameter of 3.0 cm were prepared by simple ageing method, as it has already been reported [12] (refer figure 1).
Swelling Index
The swelling index of the Chitosan films was determined by introducing a known weight of the dry Chitosan film into 1 Litre of de-ionized water in a 2 Litre jar, and was unperturbed for 24 hours. The weight of the resulting wet and swollen film is noted and the data are introduced into the following formula [13, 14].

Where, ‘W1’ and ‘W2’ Weights of Chitosan film in the dry state and the water absorbed state, respectively. SI∞ is the equilibrium swelling of the respective film. SI∞ was estimated by fitting the swelling versus time data to a second order kinetic equation of the form:

Where, SItis the swelling at time t, and kSIis a second order swelling rate constant. Swelling indices for Chitosan-Carbon ash films and Chitosan-Silica ash film was also determined similarly.
Adsorption studies
Equilibrium studies
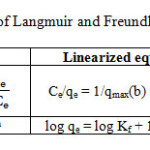 |
Table 1: Some important equations of Langmuir and Freundlich adsorption isotherms Click here to View table |
Where, ‘qmax’is the maximum monolayer adsorption capacity of the adsorbent (mg g-1),
‘KL’is the Langmuir adsorption constant (L mg-1) which is related to the free energy of adsorption
‘Ce’(mg L-1) is the equilibrium metal ion concentration.
‘KF’[mg g-1 (L mg-1)] is the adsorption capacity of the adsorbent and
‘nF’indicates favorability of the adsorption process or surface heterogeneity, provided its value lies between 1 and 10.
Adsorption of Cr(VI) from aqueous solution
A stock solution of exactly 1000 mg/L of Cr(VI) was prepared in de-ionized water using K2Cr2O7. Successive dilution was carried out for all of the working solution using required quantity of de-ionized water. The batch experiments were held out in 100mL Erlenmeyer Flasks by agitating a known quantity of Chitosan films in various temperatures. The weight of the Chitosan film adsorbents, concentrations of the Cr(VI) solution, the pH of the adsorption medium, contact time, temperature of the system were varied, for the sake of determining optimum adsorption conditions. Adsorbent films were isolated from the rest solution by a Whatmann filter paper. Adsorption isotherm studies were carried out at different initial ion concentrations of Cr(VI) from 10 to 1000 mg/L while maintaining the adsorbent dosage of 1.0 g/100 mL. The concentration of Cr(VI) was determined by a single beam spectrophotometer (systronics-106).
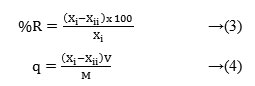
Where, ‘%R’ is the percentage removal of Cr(VI) ion, ‘Xi’ and ‘Xii’ are the concentrations of Cr(VI) metal ion before and after adsorption process, ‘V’ is the volume of the solution, ‘M’ is the weight of the respective Chitosan (or) Chitosan-ash composite film (in g) and ‘q’ is the quantity of adsorption. The linearity of response to the determination of Cr(VI) in aqueous solution using single beam spectrophotometer was r2=0.996.
Results and discussion
Characterization of Chitosan films
XRD studies
XRD studies of the Chitosan films prepared by ageing method are shown in the figures 2. It suggests that colour of the resulting films are distinguishable through naked eye. Their respective mechanical properties and FTIR studies have already been reported [14]. XRD studies revealed the amorphous nature of Chitosan films, which was retained even after the introduction of ashes. Peaks at 22.7 with free Chitosan film, 23.12 with Chitosan-Carbon ash film and 23.55 with Chitosan-Silica ash film was noted. Peaks found at almost in the similar area suggest the major properties of pure Chitosan are retained even after the inclusion of ashes. Also, the intensity of the peak with pure Chitosan is reduced in both Carbon ash inclusion and Silica ash inclusion, showing the extensive coverage of the respective ashes [15]. Another conclusion can be derived by observing the crystallinity of the peaks that, the amorphous nature of the original pure Chitosan is not disturbed.
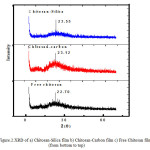 |
Figure 2: XRD of a)Chitosan-Silica film b)Chitosan-Carbon film c)Free Chitosan film (from bottom to top) |
Thermal Studies
TGA and DTA studies were used for determining the incorporation of reinforcing materials into the films. From the figures 3, it can be noted that there are two peaks one at 300°C and another at 500°C, respectively in the free Chitosan film. In the case of Chitosan-Carbon ash films, there is a notable change in the temperature region 263°C to 350°C, in the form of a steep decrease in the peak to about 50%. Whereas in the case of Chitosan-Silica ash, the decrease was noted in the temperature range of 280°C-390°C. Apart from all these, the midpoint of TGA curve has moved from 255°C to 263°C ending with 274°C, respectively, with free Chitosan, Chitosan-Carbon film and Chitosan-Silica film. The variation shows the impact of reinforcement into the network of Chitosan film.
 |
Figure 3: a)Free Chitosan fim, b)Chitosan-Carbon film, c)Chitosan-Silica film (from top left end to clockwise down) |
Swelling Index
Chitosan films were compared with Chitosan-Carbon film composite and Chitosan-Silica film composite for their efficiency to swell in an aqueous medium(refer figure 4). The aqueous medium was made absolutely free from dust and ionic impurities, by using double de-ionized water. The following conclusions were obtained after the study. There was a considerable increase in swelling quantity of the Chitosan films, when reinforcement was made with either Carbon ash or Silica ash. This ensures the fact that the reinforcing material supports the interaction of the Chitosan molecules with aqueous medium, rather than being a mere filler material. The size of the original films and that of the swollen films were compared using one feet scale.
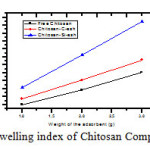 |
Figure 4: Swelling index of Chitosan Composite films |
Adsorption studies
Morphological studies
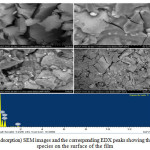 |
Figure 5: (After adsorption) SEM images and the corresponding EDX peaks showing the abundance of Cr species on the surface of the film |
Using the figure 5, it can be clearly noted that the adsorption of Cr(VI) on the Chitosan films is possible. The SEM images and their respective EDX images show the presence of Cr species on the surface of the film.
Thermodynamic studies
Thermodynamic parameters obtained for the adsorption of Cr(VI) on free Chitosan films, Chitosan-Carbon films, Chitosan-Silica films are listed in table 2.As it can be seen from the table 2, the negative values obtained for ∆H0 (-13.96, -16.94 and -25.06 KJ Mol-1), implies the exothermic nature of the adsorption process [16]. However, the values obtained for ∆S0 are positive (59.45, 69.31 and 94.32 KJ Mol-1) that obtained from ∆G0 is negative, showing the spontaneous nature of the adsorption process Cr(VI) on all three types Chitosan films.
Table 2: Thermodynamic parameters of Cr(VI) adsorption on Chitosan films
|
Type of adsorbent |
TemperatureT, in Kelvin |
1/T (K-1) x10-3 |
Equilibrium constant, Kc |
ln Kc |
∆G ̊ KJ/Mol |
∆H ̊ KJ/Mol |
∆S ̊ J/Mol |
|
free Chitosan film
|
303 |
3.30 |
5.0081 |
1.6110 |
-4.058 |
-13.96 |
59.45 |
|
308 |
3.24 |
5.5264 |
1.7095 |
-4.377 |
|||
|
313 |
3.19 |
5.9909 |
1.7902 |
-4.658 |
|||
|
318 |
3.14 |
6.5604 |
1.8810 |
-4.9730 |
|||
|
Chitosan- Carbonfilm |
303 |
3.30 |
5.0528 |
1.6199 |
-4.080 |
-16.94 |
69.31 |
|
308 |
3.24 |
5.6028 |
1.7232 |
-4.412 |
|||
|
313 |
3.19 |
6.2578 |
1.8338 |
-4.772 |
|||
|
318 |
3.14 |
6.9878 |
1.9441 |
-5.139 |
|||
|
Chitosan- Silicafilm |
303 |
3.30 |
3.9340 |
1.3696 |
-3.450 |
-25.06 |
94.06 |
|
308 |
3.24 |
4.6548 |
1.5378 |
-3.937 |
|||
|
313 |
3.19 |
5.4568 |
1.6968 |
-4.415 |
|||
|
318 |
3.14 |
6.3597 |
1.8499 |
-4.890 |
Equilibrium studies
Adsorption isotherm models were verified to evaluate the suitability of the adsorption process. For Chitosan films, Chitosan-Carbon films, Chitosan-Silica films as the adsorbent, the Langmuir model and the Freundlich model were verified. Langmuir model was verified according to the equation 5:
![]()
The corresponding Langmuir adsorption isotherms of Cr(VI) ions by Chitosan films, Chitosan-Carbon films, Chitosan-Silica films are verified. Adsorption studies were carried out by maintaining the pH of the medium at 3.0, for the overall adsorption process. For the Chitosan-Silica film the greatest equilibrium sorption capacity ‘qm’ was obtained for Cr(VI), i.e. 9.42 mgg−1, which decreased to 9.01 mgg−1 and 7.93 mgg−1 for the same Cr adsorbate when Chitosan-Carbon film were used as the adsorbent, respectively. The less agreement of the Langmuir plots with the experimental data suggests the least possibility for the monolayer coverage of Cr(VI) ions on the surface of Chitosan films, Chitosan-Carbon films, Chitosan-Silica film as the adsorbent. The corresponding values ‘qm’ and ‘b’obtained from these plots are quoted in table 3. The Freundlich parameters ‘kf’and ‘1/n’are also presented in the same table. Freundlich isotherm was verified as per the following equation [6]:
log qe = log kf + 1/n log Ce → (6)
where ‘Ce’ is the equilibrium concentration in mg l-1, qe is the amount adsorbed in mgg-1, ‘kf’ and ‘1/n’ are Freundlich constants related to adsorption capacity and intensity of adsorption, respectively. Langmuir isotherm fits best for the adsorption of Cr(VI) of Chitosan-Silica films. This is confirmed by the highest coefficient of determination (r2=0.9991).
Table 3: Langmuir isotherm parameters for the adsorption of Ni(II)on Chitosan composites
|
Name of the adsorbent |
Langmuir parameters |
Freundlich parameters |
||||||
|
‘qmax’ mg/g |
‘b’ mg/g |
Separation factor,‘RL’ |
‘r2’ |
‘n’ |
‘Kf’ |
‘r2’ |
||
|
initial conc. 50 ppm |
initial conc. 300 ppm |
|||||||
|
free Chitosan film |
7.93 |
3.7091 | 0.0053 |
0.00089 |
0.9987 | 47.84 | 7.2610 | 0.2129 |
|
Chitosan- Carbon film |
9.01 |
0.3753 | 0.0505 |
0.0088 |
0.9851 | 10.90 | 6.025 | 0.4818 |
|
Chitosan- Silica film |
9.42 |
1.4627 | 0.0134 |
0.0022 |
0.9991 | 12.52 | 6.882 | 0.9170 |
Kinetic Studies
Using table 4, the result of the kinetic studies has been reported. With the help of various Cr(VI) concentrations (50,100,150,200, 250, 300 ppm) as the adsorption medium and the fixed quantity of Chitosan films (1 g/100 mL), the concentrations of the metal ions in solution were determined at regular times.
Table 4: Kinetic study parameter for the Cr(VI) adsorption
|
Type of adsorbent |
Kinetic model
|
Concentration of Cr(VI) ppm |
r2 |
|
Free Chitosan film |
Lagergren model, pseudo I order |
50 |
0.9859 |
|
100 |
0.9886 |
||
|
150 |
0.9638 |
||
|
200 |
0.9752 |
||
|
250 |
0.9752 |
||
|
300 |
0.9416 |
||
|
Ho’s model, pseudo II order |
50 |
0.574 |
|
|
100 |
0.9368 |
||
|
150 |
0.8608 |
||
|
200 |
0.8656 |
||
|
250 |
0.8948 |
||
|
300 |
0.9178 |
||
|
Chitosan-Carbon film |
Lagergren model, pseudo I order |
50 |
0.9860 |
|
100 |
0.9866 |
||
|
150 |
0.9822 |
||
|
200 |
0.9604 |
||
|
250 |
0.9819 |
||
|
300 |
0.9645 |
||
|
Ho’s model, pseudo II order |
50 |
0.9612 |
|
|
100 |
0.9364 |
||
|
150 |
0.9574 |
||
|
200 |
0.0381 |
||
|
250 |
0.8772 |
||
|
300 |
-0.0417 |
||
|
Chitosan-Silica film |
Lagergen |
50 |
0.9909 |
|
100 |
0.9862 |
||
|
150 |
0.9645 |
||
|
200 |
0.9752 |
||
|
250 |
0.9793 |
||
|
300 |
0.9712 |
||
|
Ho’s model, pseudo II order |
50 |
0.9515 |
|
|
100 |
0.9188 |
||
|
150 |
0.9484 |
||
|
200 |
0.9105 |
||
|
250 |
0.9332 |
||
|
300 |
0.8981 |
The temperature of the system was unchanged from the room temperature and the pH of the solution was maintained at 3.0, throughout the adsorption process. The resulting data were used for the determination of kinetic model, which suits the adsorption process. The PFO and PSO models were examined. PFO and PSO models have been widely used in understanding the kinetics of adsorption. The best model was selected based on the linear regression, the coefficient of determination, r2 values. The linear equation for the PFO kinetic model of Lagergren [17] is given as;
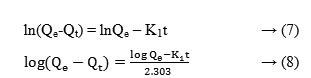
Where, ‘Qt’ is the amount of Cr(VI) adsorbed at a time (mg Cr(VI)/g of Chitosan film) and ‘k’is the equilibrium rate constant (min−1).
The Lagergren’s pseudo first order plot of log (qe−qt) versus t(minutes) for the adsorption of Cr(VI) by three types of Chitosan films as the independent adsorbents. The linearity of these plots shows that a PFO mechanism is followed in this process. The rate constants (k) for each of the systems were calculated from the linear least square method and are given in table 4 along with the corresponding coefficient of determination (r2).
Ho’s pseudo-second-order equation [8] is as given below in eqn.

The slope and intercept obtained from the linear plots of‘t/q’ versus‘t’curves were used to determine the rate constants.
The weight of the Chitosan-Carbon film and Chitosan-Silica film was considered as the weight of the free Chitosan film and it was maintained as 1.0 g. The results obtained were applied to PFO and PSO models similar to the Chitosan-Carbon film and Chitosan-Silica filmcase. The linearity of the plot obtained for PFO and PSO confirms the suitability of these mechanisms for the adsorption of Cr(VI) on Chitosan-Silica film. At lower concentrations, adsorption kinetics also fitted to PFO of Lagergren (50 ppm) together with the PSO model. Usually, for these adsorbents, the PFO kinetic model is applicable to the initial time course of the adsorption process, but, it does not represent the overall kinetic range. For all initial concentrations, the PFO kinetic model fitted better with a better co-efficient of determination and resulted in reasonable theoretical and experimental ‘qe’ values.
Effect of pH of the medium
The influence of pH of the medium on adsorption of Cr(VI) onto the Chitosan and Chitosan composites were verified by using an adsorption medium with different pH viz., pH=2 to pH=10. It was inferred from the figure 6, that the adsorption of Cr(VI) more pronounced at the acidic pH range and it was maximum at pH=3.0. The chief reason behind this fact was that anionic surfaces on the Chitosan is more efficient for adsorbing the Cr(VI), which would be in suitable disguise at this specific pH. The comparison of percentage removal of Cr(VI) using three different adsorbents implies, the greater efficiency of Chitosan-Silica composite film over the rest two adsorbent. The order of removal percentage at pH=3.0 is in the order as follows;
Chitosan-Silica composite film > Chitosan-Carbon composite film > free Chitosan film
The above comparison ensures that the anionic nature of the surface of the Chitosan films is not affected by the reinforcement of either Silica or Carbon. Rather, these reinforcement aids for the improved adsorption of the heavy metal.
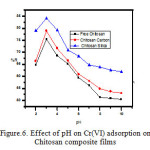 |
Figure 6: Effect of pH on Cr(VI) adsorption on Chitosan composite films |
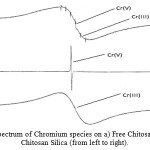 |
Figure 7: X-band ESR spectrum of Chromium species on a) Free Chitosan b) Chitosan Carbon c) Chitosan Silica (from left to right). |
ESR study of the Chromium
‘g’ values of the ESR spectrum for Cr adsorbed films was determined as 2.021, 2.022 and 2.022, respectively, for free Chitosan, Chitosan-Carbon and Chitosan-Silica films.The figure.7 shows the ESR spectrum obtained from the Cr species adsorbed on the free Chitosan, Chitosan-Carbon film, Chitosan-Silica films, respectively from top to bottom. Frequency= 9.74572 GHz, T=20°C, modulation amplitude: 2 G. Inset: t ¼ 1h, v ¼ 9.73084GHz, modulation amplitude ¼ 0.981 G, the comparison of the three Cr species adsorbed on the respective adsorbent relates the following conclusions:
Cr(VI) + CS films → Cr(V) / Chitosan films + Cr(III) / CS films
Cr(VI) + Chitosan-Carbon films →Cr(V) / Chitosan-Carbon films
Cr(VI) + Chitosan-Silica films →Cr(III) / Chitosan-Silica films
It is evident that the reduction of Cr(VI) into Cr(III) species is possible with free Chitosan and as well as Chitosan-Silica films. But in the case of free Chitosan films the interference Cr(V) species might be the reason for the decrease in the efficiency of those films [19, 20]. It can be understood that Chitosan-Carbon film is also an efficient adsorbent which acts so, by reducing Cr(VI) exclusively into Cr(V). From these discussions, the reduction of Cr(VI) species from the anionic surface of the free Chitosan, Chitosan Carbon and Chitosan-Silica films are the chief reason for the efficient removal of Cr(VI) from aqueous solution.
Conclusions
Removal Cr(VI) was performed efficiently from aqueous solution using Chitosan films and its reinforced films as the adsorbent (CS). Upon comparing CS films with the Chitosan-Silica and Chitosan-Carbon, Chitosan-Silica was found superior. The optimum pH was found to be 3.0 and optimum temperature was 30° C for Chitosan-Silica films, which ensure that the removal can be efficient in the room temperature of the Indian subcontinent. Langmuir adsorption isotherm and Pseudo First Order kinetics were followed for the adsorption process. It was found that Chitosan-Silica films reduced Cr(VI) to Cr(III) almost completely(to ≈90%).
Conflict of interest
Authors declare no conflict of interest.
References
- Frontline, August 3-16, 2002, 19(16).
- Costa, M.;Toxicol. Appl. Pharm.2003, 188, 1−5.
CrossRef - Alvarez-Ayuso, E.; Nugteren, H.W.; J.Water Res.2005, 39, 2535−2542.
CrossRef - Li, Y.J.; Gao, B.Y.; Wu, T.; Sun, D.J.; Li, W.; Wang, B.; Lu, F.; J.Water Res.2009, 43, 3067−3075.
CrossRef - Don, T.M.; King, C.F.; Chiu, W.Y.; Polym. J. 2002, 34, 418-425.
CrossRef - Ahmaruzzaman, M.D.; Adv.Colloid Interface Sci. 2008, 143, 48-67.
CrossRef
- Yingxin, Z.; Shengjiong, Y.; Dahu, D.; Jie, C.; Yingnan, Y.;Zhongfang, L.; Chuanping, F.;Zhenya, Z.Journal of Colloid and Interface Science, 2013, 395, 198–204.
CrossRef - Garg, S.K.; Tripathi, M.; Srinath, T.; Rev. Environ. Contam. Toxicol.,2012, 217, 75-140.
CrossRef - Silva, B.; Figueiredo, B.; Quintelas, C.; Neves, I.C.; Tavares, T.; Micropor. Mesopor. Mat. 2008, 116, 555-560.
CrossRef - Quintelas, C.; Fonseca, B.; Silva, B.; Figueiredo, H.; Tavares, T.; Bioresource Technol. 2009, 100, 220-226.
CrossRef - Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Chem. Eng. J.2009, 152, 440-448.
CrossRef - Abdel-Razek, A.S.; Abdel-Ghany, T.M.; Mahmoud, S.A.; El-Sheikh, H.H.; Mahmoud, M.S.; World J. Microb. Biot. 2010, 25, 2137-2145.
CrossRef - Pradhan, S.; Shukla, S.S.; Dorris, K.L.; J. Hazard. Mater. 2005, 25, 201-204.
CrossRef - Sibi, S.; Pragathiswaran, C.; Venkatesan, S.; Anthuvan Babu, S.; Sundaram, T.T.; Subramani,K.; Int.J.Curr.Res.Chem.Pharma.Sci., 2014, 1(6),154-163.
- Deng, K.; Zhang, Y.; Jia, N.; Liu, J.; Zheng, X.; Tian, H.; Chemical Journal on Internet, 2007, 9, 19-30.
- Langmuir, I. J. Am. Chem. Soc., 1918, 40(9), 1361-1403.
CrossRef - Lagergren, S. Handlingar, 1898, 24, 1-39.
- Ho, Y.S. Scientometrics, 2004, 59,171-177.
CrossRef - Sebastian, B.; Silvia, G.; Juan, C.G.; Ana, M.A.; Luis, F.S.; Sandra, S.; Separation Science and Technology, 2008, 43(11), 257-287.
- Gregory, B.D.; Mary, C.; Rachel, C.; Rodney, P. F.; Jennifer, A.I.;Peter, A.L.J. Chem. Soc. Faraday Trans., 1995, 91(8),1207-1216.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









