Adsorption of Methyl Green onto Zeolite ZSM-5(pyrr.) in aqueous solution
Maaza Lamia*1, Djafri Fatiha2, Bouchekara Mohammed1 and Djafri Ayada3
DOI : http://dx.doi.org/10.13005/ojc/320118
Article Received on :
Article Accepted on :
Article Published : 05 Mar 2016
In this work we report the preparation of ZSM-5 (pyrr) with molar composition: 0.2057 Na2O - 0.00266 Al2O3 – SiO2 –0.68 Pyrr (pyrrolidine) - 40 H2O – 0.12H2SO4by hydrothermal synthesis method. The sample is characterized using IR spectra, MEB and XRD. The removal of Methyl Green (MG) dye from aqueous solutions onto ZSM-5(pyrr.), is examined under various experimental conditions (dyes concentration, amount H-ZSM-5(pyrr.), temperature, pH and contact times).The adsorbed amount increased with increasing amount dye, amount H-ZSM-5(pyrr.), time, temperature and PH. The linear correlations of Langmuir and Freundlich isotherms are determined. The experimental results show that we have model Langmuir. Free energy ∆G° enthalpy ∆H°, entropy ∆S° and activation energy Ea are determined. The ∆H°, ∆G° andEa values indicated that the adsorption of methyl green dye onto H- ZSM-5(pyrr.) is exothermicphysisorption process. Kinetics analyses are conducted using pseudo first-order, second-order and the intraparticle diffusion models. The results showed that the adsorption kinetics was controlled by a pseudo second-order model.
KEYWORDS:Hydrothermal synthesis; zeolithe ZSM-5; Isotherm adsorption; Methyl green; IR; SEM
Download this article as:| Copy the following to cite this article: Lamia M, Fatiha D, Mohammed B, Ayada D. Adsorption of Methyl Green onto Zeolite ZSM-5(pyrr.) in aqueous solution. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Lamia M, Fatiha D, Mohammed B, Ayada D. Adsorption of Methyl Green onto Zeolite ZSM-5(pyrr.) in aqueous solution. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14691 |
Introduction
During the past decades, the pollution of the environment becomes a serious problem in the world. Numerous chemical compounds have been recognized as poisonous or dangerous. Various techniques have been developed in investigation of processes of pollutant removal or degradation. It is known that liquid-phase adsorption is one of the most efficient methods for the removal of colors and organic pollutants. The usage of solid materials is necessary in this procedure. Various porous materials are used as adsorbents in adsorption processes. Solid materials as zeolites are used for the removal of pollutants that can be found in wastewaters. Zeolites are hydrated porous aluminosilicates. Their structures consist of a three-dimensional framework into which organic compounds (phenols, synthetic Dyes…) may be adsorbed. As part of our research on porous materials, we present the synthesis of ZSM-5 in hydrothermal conditions with structuring pyrrolidine agent and we examined its adsorption ability of Methyl Green dye, using UV-Visible spectrophotometer technique.
Materials and methods
Synthesis of Zeolite
Zeolite ZSM-5 , MFI-type is synthesized according to published method1,2 from a mixture of 0.823g of sodium hydroxide in aqueous solution, mineralizing agent, 0.1g aluminium nitrate, 7.5g of silica (Ludox-AS-40) 2.4g of pyrrolidine, structuring agent (C4H9N (Merck)) and 0.6g of H2SO4 . This mixture is stirring for about 1h30 until obtaining a clear gel (pH=12) which is introduced into a stainless steel autoclave. The autoclave was placed in the oven at a temperature of 150°C for a period of 5 days. On leaving the oven, the autoclave is cooled rapidly under running water. The solid is collected by Büchner filtration, washed several times with cold distilled water, and then dried in an oven at 100°C for 12 hours. Using NaOH as a mobilizing agent, fine, white micro crystalline powder is obtained . The Na- ZSM-5 iscalcined for 6h in atmospheric air at 500°C. The hydrogen form zeolite (H- ZSM-5(pyrr.) is obtained with adding 20 ml of ammonium chloride (0.6mol/l) to 1g of Na-ZSM-5 with stirring at 60°C during 2h; the ammonium-zeolite is washed with dezioned water and calcined at 500°C for 6h.
The elaborated H-ZSM-5 is characterized by IR Spectra, MEB (JEOL JSM-6610LA) and X Ray Diffraction..IR Spectra, taken in Brüker Vector 22 spectrophotometer is reported in a range of frequency 500–4000 cm-1. The XR diffractograms are recorded on Brücker AXS D8 Advance diffractometer with a graphite monochromator, using a Cu Kα radiation (λ =1.541 Å). The centered peaks 2θ are observed in a range of 6 and 50 °.
Adsorption study of organic dye in aqueous solutions
Dyes dissolved in water, are classified as acid and basic dyes.Aqueous solution of Methyl Green or anion MG has λmax = 632nm and value. Before using, MG dye is dried at 110oC for few hours. The stock solution of MG is prepared by dissolving 1g of dye in 1L of de-ionized water to give the concentration of 103mg.L-1. The solution of MG is blue turkey in color. Several dilutions are prepared by diluting mother dye solution to give standard solutions in the study concentration range.
To determine the effects of dye concentration, the dye concentrations used, are 10mg.L-1, 20mg.L-1,30mg.L-1, 40mg.L-1 and 1g.L-1 of zeolite and the contact times used in the adsorption process are 10,20,30,60, 90,120,180,300 and 900mn. The effect of pH is examined in the range 2-9 for GM at 25oC. To examine the effect of the temperature in the range 20-90oC, the amounts of the zeolite used are m1=10-2 and m2=15.10-3 mg and the dye concentration is 40mg.L-1.To determine the effect of adsorbent dosage, the zeolite mass used, is m1=10-2, m2=15.10-3, m3=2. 10-2 and m4=3.10-2mg/g with dye concentration of 40mg. L-1.
In experimental process, an amount of adsorbent ZSM-5(pyrr.) is placed in a borosilicate glass reactor of 100ml capacity, containing aqueous diluted solutions of known dye concentrations. Inserted in a water bath to maintain the temperature constant, the reactor is vigorously shaken with constant speed of rotation 350rpm.
The liquid and solid phases are separated by filtration. The uncolored dye solution is analyzed to determine the removal MG concentrations by UV–visible spectrophotometer (UV-Vis NV202) at wavelength for maximum absorbance (λ max).Absorbance of color dye without absorbent is controlled to so that the absorption process is due to the absorbent and not the nature of the reactor.All experiments are carried out by changing one parameter and conducted in duplicate in the same conditions.
The percentage removal dye Cads defined as the ratio of difference in dye concentration before and after absorption (Co -Ct) where Co and Ct are respectively the initial concentration and the concentration at time t of dye calculated from absorbance of aqueous solution is calculated using the equation:
% Cadsb = 100.(Co – Ct ) /Co (eq.1)
The amounts of dye adsorbed at equilibrium per unit mass of the adsorbent qe (mg.g-1) are calculated from the equation:
qe= V. (Co-Ce)/W (eq.2)
Where Co and Ce are respectively the initial and equilibrium concentrations dye (mg.L-1), V the volume of the solution (L) and W the mass (g) of the zeolite.
Results and discussion
Characterization of prepared H- ZSM-5(pyrr).
Infra-red absorption spectra
IR spectra of zeolite H-ZSM-5(pyrr.) is shown in figure 1. The wave bands at 437.01cm-1, 563.01 cm-1, 788.79 cm-1, 912.84 cm-1, 1043.78 cm-1 and 1212.38 cm-1 are detected. The two characteristic absorption bands 1212.38 cm-1 and 563.O1 cm-1 are assigned to the vibration of an oxygen five-membered ring3. It is proposed in the literature that certains oxygen aluminosilicalitepolyanions whose structure consist of five-membered rings, are stabilized in pyrrolidine system.
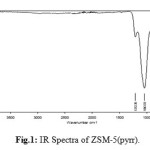 |
Figure 1: IR Spectra of ZSM-5(pyrr). Click here to View figure |
The asymmetric stretching vibration and the vibration of stretching/bending symmetry of the Si-O-Si bridge are characterized respectively by 1043.78 cm-1, 1212.38 cm-1 and 912.84 cm-1. Absorption weak and low intense peak at respectively 437.01cm-1 and563.01 cm-1 are attributed to rocking vibration of Si-O-Si4. The wave length situated at 788.79 cm-1 indicated low presence of morphed phase
The X-Ray powder diffractogram
XR diffractogram of the zeolite are presented in Figure 2. The analysis of the diffractogram demonstrates the presence of peaks (2θ) located between 7 and 50 degree. The peaks centered at 2θ =7.98, 8.94, 23.06 and 23.34 degrees confirms the presence of zeolite type MFI, ZSM-5. Around 2θ= 21° we observe a low intense peak which indicates the presence of low amount of amorphous phase.
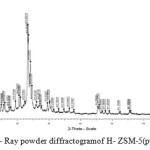 |
Figure 2: X- Ray powder diffractogramof H- ZSM-5(pyrr). |
Scanning Electron Microscopy (SEM)
Figures 3a and 3b indicate the crystal morphology of zeolite which is characterized by round edged-hexahedrons. Figure 3a shows little amount of amorphous phase closer the edged form which confirms XRD spectra . In Figure 4 b, MG dye is no homogeneously dispersed in zeolite.
Adsorption of dye
In adsorption, in first state the solute adsorbate is transferred from the aqueous solution to the adsorbent surface(physicochemical process). The adsorption of dye onto zeolite depends on several factors:
Effect of pH
The adsorption capacity of anionic or cationic dye depends on the pH of the solution. The impact of initial pH on adsorption capacity of GM dye (40mg.L-1) onto H- ZSM-5(pyrr.) is investigated with 0.01g adsorbent at the temperature of 25 °C over the range 5–12 of pH (Table1).
Table 1: Effect of pH on adsorption capacity of GM dye onto H-ZSM-5(pyrr).
| pH |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
| Q(mg/g) |
5.36 |
26.84 |
31.38 |
33.52 |
35.2 |
36 |
38 |
38.3 |
38.6 |
39.2 |
39.48 |
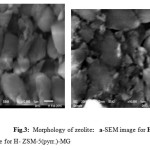 |
Figure 3: Morphology of zeolite: a-SEM image for H-ZSM-5(pyrr.) b-SEM image for H- ZSM-5(pyrr.)-MG |
In acidic solution from pH<7, the increasing adsorption of GM is observed, which decreases drastically in the range7-9 (fig.4).This phenomena is explained by the electro-static attraction between anionic dye(GM),the positive surface charge and/or Lewis acid site of the zeolite. On the other hand at higher pH>7, basic solution, the decreasing of removal anionic dye (GM) is due to electrostatic repulsion between the negatively charged surface and dye molecules.
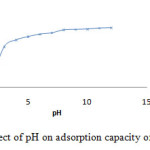 |
Figure 4: Effect of pH on adsorption capacity of GM dye. |
Effect of initial concentration dye and contact times
It is apparent from Figure 5 , that in the first 20 min times, the adsorption capacity of removal dye from solution increased rapidly, after that, it increased slowly till saturation is allowed at time of 180mn which is not affected by the initial concentrations dye. In the other hand, high initial concentration dye shows better adsorption performance.
Initially, in first step, in the adsorption process all adsorbent sites are vacant, so the adsorption is high. In the second step, with the decreased in the number of adsorption sites on the zeolite, and the decrease of dye concentration, the adsorption became slow which is explained by the repulsion between free molecule dye present in the liquide phase and located dye molecules and/or the vacant sites are difficult to attain5.
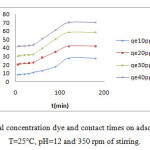 |
Figure 5: Effect of initial concentration dye and contact times on adsorption of GM dye at T=25°C, pH=12 and 350 rpm of stirring. |
Effect of temperature on removal dyes
The thermal variation of the adsorption of MG onto zeolite is investigated over the temperature range 20-90oC. Generally the temperature influences the adsorption equilibrium. The increasing of temperature reduces the viscosity of the solution, which increases the solubility of color dye in liquid phase and its diffusion rate in the pores of the Zeolite. In our study, the figure 6 indicates the increasing of the adsorption capacity with temperature which indicating exothermic adsorption of GM dye onto H- ZSM-5(pyrr.).
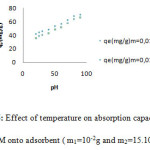 |
Figure 6: Effect of temperature on absorption capacity of GM onto adsorbent ( m1=10-2g and m2=15.103g) |
Effect of the zeolite mass and contact times on the adsorption of 40mg.L-1 GM dye
The decreasing of adsorption capacity of MG dye with the increasing of adsorbent zeolite is shown in figure7.This phenomena is probably due to the fact that some of the adsorption sites remained unsaturated during the process6. In all cases, adsorption equilibrium is attained at 180mn after which the adsorption capacity of removal dye decreases and saturation of active sites take place. The presence of a higher number of adsorption sites on a small mass of the dye, will be also taken in account for this effect.The highest percentage removal of MG is 70.05for 10-2mg/g of sorbent. After 15h the removal dye is the same obtained at 20min of adsorption.
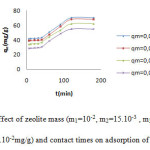 |
Figure 7: Effect of zeolite mass (m1=10-2, m2=15.10-3 , m3=2.10-2 and m4=3.10-2mg/g) and contact times on adsorption of MG dye. |
Modeling of the isotherms adsorption
Adsorption is a physicochemical process that involves mass transfer of a solute from liquid phase to the adsorbent surface. Kinetic study provided important information about the mechanism of color dyes adsorption onto ZSM-5(pyrr.).
Langmuir7, Freundlich8 and intra-particle diffusion isotherms have been selected for studying the adsorption. Linearized Langmuir isotherm equation 3 and Freundlich isotherm equation 4 can be expressed as follows:
qe=b.qmaxCe/1+bCe(Eq.3)
Where qmax (mg/g) is the maximum amount of dye adsorbed per unit mass of the adsorbent required for monolayer coverage of the surface and b thermodynamic constant of adsorption equilibrium (m/mg).
lnqe= ln KF + (1/ RL) ln Ce (Eq.4)
Where qe is the amount adsorbed at equilibrium (mg/g) and Ce(mg/L) is the equilibrium concentration of MG, KF and RL are Freundlich constants, where KF (mg/g (L.mg-1) 1/ RL) is the adsorption capacity of the adsorbent and RL giving an indication of how favorable the adsorption process.
The Langmuir model (fig.8a) assumes that adsorption takes place at uniform energy sites on the adsorbent surface and Freundlich isotherm model(fig.8b), empirical relationship assumes that different sites with several adsorption energies are involved. When Ce/qe is plotted against Ce, a straight line with a slope of 1/qmaxis obtained, as shown in Figure 8a, indicating that the adsorption of methyl green on zeolite follows the Langmuir isotherm. The Langmuir constants KL and qmax are calculated from this isotherm and their values are listed in Table 2.
 |
Figure 8: a- Langmuir plot for adsorption of MG onto H- ZSM-5. b- Freundlich plot for adsorption of MG onto H-ZSM-5. |
Langmuir and Freundlich constants (Table2) for adsorption of MG onto H-ZSM-5 indicate the R2 values are higher than 0.99 and that monolayer coverage of the dye is adsorbed on the surface of H- ZSM-5(pyrr).
Table 2: Langmuir and Freundlich constants for adsorption of MG onto H-ZSM-5
|
Langmuir Model type 1 |
Freundlich Model |
| R2=0.9922 | Kf=11.17 |
| qMAX=70.084 mg/g | R2=0.9838 |
| Kl=0.0257 | n=2 |
Thermodynamic parameters
The thermodynamic parameters for the adsorption of MG bye ZSM-5 such as the enthalpy change (ΔHo), the Gibbs free energy change (ΔGo) and the entropy change (ΔSo); adsorption of MG from ZSM-5 may be related to the coefficient of distribution of solutions between the solid phase and the solution phase by the following equation 9,10
ΔS° = ( ΔH°ads / T – ΔG° / T ) (Eq. 5)
LnKd = LnC0 = – ΔG° / RT (Eq.6)
LnKd = LnC0 = ΔS° / R – ΔH°ads / RT (Eq.7)
The values of the distribution coefficient Kc are reported in table 3 and the right of the function lnKd = f (1 / T) is shown in Figure9.
Table 3: The values of the distribution coefficient Kd
| 1/T (°K) | 0.0034 | 0.003335 | 0. 0033 | 0.00319 | 0.00309 | 0.003 | 0.0029 | 0.0028 | 0.00275 |
| ln Kd | 0.0103 | 0.0105 | 0.0114 | 0.0120 | 0.0133 | 0.0142 | 0.0155 | 0.0171 | 0.0176 |
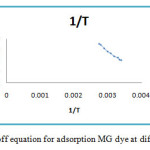 |
Figure 9: Van’t Hoff equation for adsorption MG dye at different temperatures. Click here to View figure |
The value of the Gibbs energy for the different temperatures is lessthanzero (ΔG0<0), which proves that the process of elimination of MG in the zeolite solution is instantaneous10, 11. The calculated values of the enthalpy at different temperatures are alsolowerzero (ΔH0<0), the negative ΔH0 value (table 4) indicating that this process is exothermic12 , 13. The negative value of entropy, reflecting no significant change is produced in the internal structure of the zeolite in the adsorption of thesemetal ions14, 15. It isnotedalso for bothadsorbents, ΔG°decreases with increasing temperature of the solution. This can be explained by the fact that adsorption becomes increasingly with the dehydrated anions at high temperature.
Table 4: The thermodynamic parameters for the adsorption of MG dye onto ZSM-5(pyrr.)
| dye | T(K) | ΔG(Kj,mol-1) | ΔadsH(Kj,mol-1) | ΔadsS(j,k-1,mol-1) | R2 | |
|
293 |
-25.09 |
-25.13 |
-0.0001337 |
|||
|
298 |
-26.01 |
-26.06 |
-0.000167 |
|||
| MethylGreen |
303 |
-28.86 |
28.92 |
-0.000199 |
||
|
313 |
-31.38 |
-31.43 |
-0.000148 |
0.991 |
||
|
323 |
-35.85 |
-35.9 |
-0.000154 |
|||
|
333 |
-39.31 |
-39.37 |
-0.000169 |
|||
|
343 |
-44.2 |
-44.265 |
-0.000185 |
|||
|
353 |
-50.3 |
-50.37 |
-0.000189 |
|||
|
363 |
-53.23 |
-53.3 |
-0.000172 |
|||
Evaluation of adsorption kinetics
Two of the widely used kinetics models, pseudo first-order16 and pseudo-second-order17 are used to examine the adsorption kinetics of MG onto ZSM-5(pyrr.). The select model is based on the regression correlation coefficient values (R2). The pseudo -first and second -order kinetics model might be represented respectively by the following equations:
ln (qe-qt)/qe=-k1t (Eq.8)
t/qt=1/2k2qe2+t/qe (Eq.9)
The pseudo first and second -order kinetics model are represented by figure 10 and figure 11.
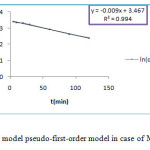 |
Figure 10: Kinetics model pseudo-first-order model in case of MG onto ZSM-5 |
To calculate the activation energy of the adsorption process, Arrhenius equation is adopted, with the rate constants (k1 and k2) of the pseudo-first and second-order models18.
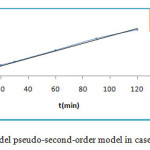 |
Figure 11: Kinetics model pseudo-second-order model in case of MG onto ZSM-5 Click here to View figure |
ln(ki) =ln(A) –Ea/RT (Eq.10)
where ki, A, Ea, R and T are the rate constants of the pseudo-first-order model (min-1) or second-order model.
Table 5:Parameters of pseudo-first and second-order kinetics models of removal MG
| Pseudo-first order model | Pseudo-second order model | ||||||||
| qexp | qthe | k1(min-1) | R2 | t1/2 | k2(min1.g/mg) | R2 | t1/2 | qthe | Qexp |
| 32.27 | 70.5 | 0.00705 | 0.995 | 98.31 | 0,0164 | 0.994 | 1.32 | 70.05 | 137.93 |
The correlation coefficient for pseudo-first-order kinetic model R2 is sleety greater than 0.98, values qe,cal and qe,exp as shown in table 5, are very different but for the second-order kinetics model R2 value is more than 0.99 . According to Langmuir equation the maximum capacity of MG dye adsorption (qmax) is obtained as 35.44 mg/g at 25°C. Thus indicated that the kinetics of the adsorption MG dye could be described by the pseudo-second-order kinetics model, process indicating that the formation of a monolayer of dye molecules is predominant.
Intraparticle diffusion model for MG
The Intraparticle diffusion model is proposed by the equation19.
qe= ki t ½ +C (Eq.11)
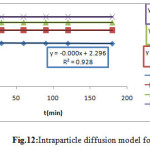 |
Figure 12: Intraparticle diffusion model for MG |
In the figure12 all the linear portions did not pass through the origin, thus indicated that intra-particle diffusion is not the only rate-controlling step (18)19.
The important parameter RL20 constant (Table9) referred to as equilibrium parameteris calculated from equation:
RL = 1/ (1 + KL Co) (Eq.12)
Where KL is the Langmuir constant (L.mg-1) and Co is the highest initial dye concentration (mg.L-1). Value RL gives an indication of how favorable the adsorption process.
Table 6: RL values for different concentrations
| C (ppm) |
10 |
20 |
30 |
40 |
| RL |
0.00255 |
0.0011 |
0.00068 |
0.00047 |
The isotherm is unfavorable with RL>1, linear withRL = 1, irreversible with RL = 0 and favorable with 0< RL<1. As shown in table 9, RL values of MG adsorption onto H- ZSM-5(pyrr.)calculated are between one and zero, which indicated favorable adsorption21.22 (Table 6).
Conclusion
In this study, H-ZSM-5(pyrr.) is prepared by hydrothermal method and characterized by IR spectra, SEM and XRD. The performance of adsorbed Methyl Green Dye is examined. The amount of Methyl Green dye adsorbed per unit of zeolite mass increased with increasing initial dye, adsorbent concentrations and temperature. The adsorption kinetics of Methyl Green onto H- ZSM-5(pyrr.) is controlled by the pseudo second-order model. The equilibrium adsorption data fit to the Langmuir isotherm.The thermodynamics of the adsorption process revealed that it is an exothermic, spontaneous and physisorptionprocess.
References
- Lagergren, S.Handlingar.1898, 24, 1-39.
- Suzuki,K.; Kiyozumi, Y.; Shin, S.; Fujisawa, K.; Watanabe, H.; Saito, K.; Noguchi, K.J .Zeolites.1986, 6, 290-298.
CrossRef - Kokotailo, G.T.; Lawton, S.L.; Olson D.H.; Meier, W.M. Nature.1978, 272, 437-438.
CrossRef - Flaningen, E.M.; Khatami, H.; Szymanski, H.A.Adv.Chem. Ser.1971, 101, 201-229.
CrossRef - Mall, ID; Srivastava V.C.; Agarwal, N.K. Dyes Pigment.2006, 69, 210–223.
CrossRef - Khatod,I. J. Chem. Res.2013, 5, 572-577
- Langmuir, I. J. Am. Chem. Soc.1918,40, 1361–1403.
CrossRef - Adebowale, K.O.;Unuabonah I.E. ;Olu-Owolabi, B.I.Journal ofHazardousMaterials B.2006, 134, 130 – 139.
- Seki, Y.; Yurdakoc¸ K. Adsorption.2006,12, 89–100.
CrossRef - Tunali, S.; Akar.T. Journal of HazardousMaterials.2006,131, 137 – 145.
CrossRef - Dubey, S.P. ;Gopal, K.Journal of HazardousMaterials.2009, 164, 968 – 975.
CrossRef
- Sarý, A. ;Tuzen, M. Journal ofHazardous Materials.2009, 164,1004 –1011.
CrossRef - Yu.Y.; Zhuang, Y.Y. ; Wang, Z.H.Journal of Colloid and Interface Science.2001, 242, 288 – 293.
CrossRef - Ada, K.; Tan, S. ;Ergene, A. ; Yalcin, E.Journal of HazardousMaterials.2009, 165, 637 – 644.
CrossRef - Alkan, M.; Demirbas, Ö. ; Dogan, M.Micropor and MesoporMaterials.2007, 101, 388-396.
CrossRef
- Demirbas, E.; Dizge, N.; Sulak, M.; Kobya, M.Chem. Eng.2009,148, 480–487.
CrossRef - Feng, Z.; Shao, Z.; Yao, J.; Huang, Y.; Chen, X.Polymer. 2009, 50, 1257–1263.
CrossRef - Doan, M.; Alkan, M. Journal of Nanostructure in Chimistry.2003, 50, 517–528.
- Weber, W.; Morris, J.Journal of the Sanitary Engineering Division.1963, 89, 31–59.
- Ho, Y.S.; McKay, G.Process Biochemistry.2003,38, 1047–1061.
CrossRef - Kumar, A.; Kumar, Sh.; Kumar, Su. ; Dharame, V.G. J. of Hazardous materials.2007, 147, 155-166.
CrossRef - Aksu. Z.ProcessBiochemistry.2002,38, 89 – 99.

This work is licensed under a Creative Commons Attribution 4.0 International License.









