Process intensification approach for the synthesis of metal nanoparticles : A Mini Review
Bahareh Khodashenas* , Ramin Zadghaffari and Sobhan Dafe Jafari
Department of Chemical Engineering , Ahar Branch, Islamic Azad University, Ahar , Iran. Corresponding Author Email: bahar.khodashenas67@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/31.Special-Issue1.30
Article Received on :
Article Accepted on :
Article Published : 10 Sep 2015
Nowadays nanoparticles are of great interest of researchers because of their various applications in different fields. Since, using the intensification technology which, includes minimization plant or equipment size in the synthesis process, leads the process miniaturization, energy efficiency, safety and cost reduction using this technology in the production of nanoparticles is highly regarded. The present paper, is an attempt to present a review of Process intensification (PI) techniques which are used in nanoparticles synthesis process.
KEYWORDS:Intensification; Nanoparticles; Synthesis; SFTR; Microreactor; SDR
Download this article as:| Copy the following to cite this article: Khodashenas B, Zadghaffari R, Jafari S. D. Process intensification approach for the synthesis of metal nanoparticles : A Mini Review. Orient J Chem 2015;31(Special Issue1). |
| Copy the following to cite this URL: Khodashenas B, Zadghaffari R, Jafari S. D. Process intensification approach for the synthesis of metal nanoparticles : A Mini Review. Orient J Chem 2015;31(Special Issue1). Available from: http://www.orientjchem.org/?p=10756 |
Introduction
Nanotechnology is the science of the small; the very small. It is the use and manipulation of matter at a tiny scale. At this size, atoms and molecules work differently, and provide a variety of surprising and interesting uses 1. Nanotechnology represents the design, production and application of materials at atomic , molecular and macromolecular scales, in order to produce new nano-sized materials 2. It is predicted that in the 21st century, nanotechnology will significantly influence science, economy and daily life and will become one of the driving forces of the next industrial revolution 3. Nanomaterials often show unique and considerably changed physical, chemical and biological properties compared to their macro-scaled counter parts 4. Nanoparticles are defined as particulate dispersions or solid particles with a size in the range of 10-100nm 5.
The present review is aimed at reviewing the research carried out in the field of process intensification in nanoparticles production. The Segmented Flow Tubular Reactor (SFTR), Microreactor, and spinning disc reactor (SDR) with their special characteristics are very potent in gaining access to nanoparticles.
Process Intensification (PI)
One of the earliest references to intensification of processes was in a paper published in the US in 1925 6.The concept of process intensification was first introduced by Ramshaw 7. Keller and Bryan (2000) , highlighted the fact that growing worldwide competition will necessitate major changes in the way plants are designed. These authors expressed seven key themes that would mould developments in industry. The key themes are mentioned below 8,9.
- Capital investment reduction
- Energy use reduction
- Raw material cost reduction
- Increased process flexibility and inventory reduction
- Ever greater emphasis on process safety
- Increased attention to quality
- Better environmental performance
Process intensification aims to make dramatic reductions in plant volume, ideally between 100- and 1000-fold, by replacing the traditional unit operations with novel, usually very compact designs, often by combining two or more traditional operations in one hybrid unit , to develop and use multifunctional devices for performing heat and mass transfer, separation, extraction and mixing operations 7,10. In order to develop the Process intensification, all the operation systems such as : reactors, mixers, heat exchangers, distillation columns, separators and etc., are required to be intensified 7. Figure 1 shows the classification of PI equipment and methods. Process intensification (PI) has four goals: 1. To maximize the effectiveness of intra- and intermolecular effects, 2 & 3. To optimize the driving forces/maximize specific interfacial areas, and 4. to maximize the synergistic effects of partial processes 11.
There are two major objectives for process intensification: 1. Process miniaturization: To achieve process miniaturization, two different approaches can be employed. The first approach involves reduction in equipment size through higher intensities for mixing and heat and mass transfer, such as centrifugal fields for example. The second approach involves reduction in equipment size through the use of smaller scales of internal structure, such as micro reactors and 2. Process integration: to achieve process integration, multiple process tasks can be combined in a single piece of equipment For example the combination of reaction and separation steps in the same equipment 12. The advantages of PI is shown in Fig. 2.
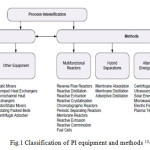 |
Figure 1: Classification of PI equipment and methods 13, 14 Click here to View figure |
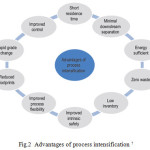 |
Figure 2: Advantages of process intensification 7 Click here to View figure |
Using Process intensification in the Synthesis Of Nanoparticles
Nanoparticles are the simplest form of structures with sizes in the range of 1-100 nm. Nanomaterials may provide solutions to technological and environmental challenges in the areas of solar energy conversion, catalysis, medicine, and water treatment 15. Nowadays, metallic nanoparticles are highly regarded because of their wide range of applications which lets their uses in different industries16. Generally, the synthesis of nanoparticles is carried out through physical, chemical and biological methods. These methods can be used to prepare nanoparticles of different diameter and morphology by controlling reaction conditions. There are various types of physical and chemical methods in order to produce nanoparticles but some of these methods are expensive or use toxic substances 17.
Among various NPs, metal NPs have been highly considered by researchers due to their antibacterial properties that result from their high surface area to volume ratio and also the resistance of microbial growth against metal ions, antibiotics and resistant strains development. Change in the size or surface area of the composition can change the physical and chemical features of the NPs. Silver NPs are examples of metal NPs, which have attracted much attention because of their application in various industries and sciences: i.e. in medicine, in the food industry, as a catalyst, in chemical reactions and many other fields, which is a result of their catalytic, electronic, optical and also antibacterial properties 18. The shape, size and size distribution of silver nanoparticles can be controlled by adjusting the reaction conditions such as reducing agent, stabilizer and different synthetic methods. In the past few years, different process intensification technologies are used for the preparation of nanoparticles 3,19.
One of the oldest technologies in process intensification is the high gravity, (HIGEE) contactor which is also known as rotating packed bed (RPB), developed by Ramshaw in 1979, where the packing in a packed bed rotates (RPB) at high speed to give high acceleration to liquid (of the magnitude of 2000 – 1000 m s -2 ). The phenomenon of forcing liquid out towards the periphery of the bed forms thin films over the packing and leads to high mass transfer coefficient 7,20. HIGEE technology is used for different applications, e.g., nanoparticle synthesis, polymerization, deoxygenation of water, desulfurization, etc 7.
Segmented Flow Tubular Reactor (SFTR)
The SFTR concept was revealed at the École Polytechnique Fédérale de Lausanne (EPFL) on 15 July 1996 when the invention was disclosed in a patent application 21. The main motivation was the development of a new technology devoted to overcome the main limitations associated to the mass and heat transport issues during the scale up of high-tech laboratory chemical production in the form of sub-micrometric powders. In fact, although many excellent powders have been discovered and prepared in laboratories at the mg level, transferring these processes to the kg production scale is often a bottleneck that hinders the creation of new innovative materials 21. The SFTR has been developed to overcome the classical problems of powder production scale-up from batch processes, which are mainly linked with mixing, homogeneity, and heat transfer. The SFTR is composed of three distinct parts: a micromixer which ensures that the coreactants are efficiently mixed, a segmenter, and a tubular reactor, placed in a thermostatic bath. Aimable et.al carried out investigation on nanoparticles and their application in Segmented Flow Tubular Reactor (SFTR). They presented process intensification using a segmented flow tubular reactor (SFTR) for ultrafine CaCO3, BaTiO3, and nanosized ZnO from optimized minibatch (20 mL) conditions. With SFTR, it was possible to scale out the powder production from very low optimization volumes (40 cm3) at the laboratory scale without changes in powder quality. They concluded that the SFTR was a powerful tool for the production of powders and nanocrystals 22. An investigation on precipitation system for zinc oxide and aluminum doped zinc oxide nanoparticles was carried out by Aimable et.al. They used mild hydrothermal conditions by using the Segmented Flow Tubular Reactor (SFTR) for synthesis of zinc oxide nanoparticles. Microwave assisted hydrothermal process was having advantage of directly forming fully crystalline powder. It was also observed that with addition of aluminum and polyacrylic acid, a better control on size and morphology was possible 23. Figure 3 is a schematic view of the SFTR and its components.
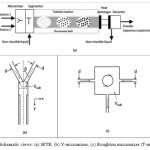 |
Figure 3: Schematic views: (a) SFTR, (b) Y-micromixer, (c) Roughton micromixer (T-mixer) 22 Click here to View figure |
Microreactor / Continuous flow microreactor
Microreactors—also known as micro-structured reactors or micro-channel reactors—are very small devices, with channel dimensions of less than 1 mm, in which chemical reactions take place 24. Microfluidic devices are process intensifying devices, which offer several advantages over batch processes for the production of nanoparticles. The large surface area to volume ratio of microreactors offers enhanced heat and mass transfers in comparison with conventional batch reactors. This intensification occurs in microfluidic devices mainly because of control over heat transfer and effective mixing due to the reduced characteristic time of the transport processes. Moreover, efficient mixing is a key advantage in preparing narrow size distribution of nanoparticles in microreactors. In all, microreactors offer many opportunities for exploring and developing novel nanoparticle synthesis routes. Therefore, nanoparticles with a certain particle size, uniform size distribution, and desired structure can be generated through efficiently controlled reactions in microreactors 25,26,27. Microreactors can be classified to continuous flow 28,29 and droplet-based or segmented flow 30,31 based on their flow type. Microreactors can also be classified based on the material they are made out of (such as : silicon 32, polymers 33, metals , ceramics (including glass and fused silica) 34, Glass-substrate microreactors 35 and reactors made out of capillary tubes 36 ). Three types of reaction consist of : Liquid-phase reactions, Liquid–solid phase reactions and Liquid–gas reactions can be carried out in microreactor 34.
Wagner et al. (2005) synthesized gold nanoparticles in the size range of 5 to 50 nm from a gold salt (HAuCl4) and a reducing agent (ascorbic acid) in a microreactor 37. Singh et al., (2009) could synthesize gold and silver nanoparticles using a polydimethylsiloxane (PDMS) in microreactor by reduction reaction between metal salt solutions and borohydride with tri-sodium citrate as the capping agent 38. Wagner et al. (2004) synthesized spherical gold nanoparticles with the size range of 5-50 nm by using gold precursor and ascorbic acid, and they have used different flow rates. They found that by doubling the flow rate a reduction in narrow size is achieved in comparison to batch synthesis 39. Patil et al., (2012) Synthesized silver nanoparticles in microreactor and batch reactor by using sodium borohydride and silver nitrate. Two surfactants namely sodium dodecyl sulfate (SDS) and N-cetyl N,N,N,trimethyl ammonium bromide (CTAB) were used to evaluate the effect on particle size by controlling nucleation and growth mechanism. The optimum conditions and parameters for microreactor was identified and maintained. Microreactor in which AgNO3 flow rate was 1 mL/min (0.001 M) and NaBH4 was 3 mL/min (0.003 M) shows minimum particle size of 4.8 nm 40.
Xu et al., 2015 could synthesize nickel nanoparticles with hydrazine hydrate as the reducing agent in a T-shaped continuous flow microreactor at 80 ˚C . Nickel nanoparticles have attracted a large amount of interest for their magnetic and catalytic properties due to the abundance of Ni compared to other group metals, which inspires research on size and shape control of nanostructures. Microreactors have gained much attention in the field of materials preparation due to the possibility of precise control of reaction and mixing conditions. The benefits from microreactors are high-surface-area-to-volume ratio, tunable inner-wall proper- ties, flow orientation and flexible-structure designs of the micro- mixers. Microfluidic reactors in comparison with bulk batch reactors can control the reaction more precisely which exhibit relatively poor mixing and mass transfer performance. Compared to bulk batch reactors, continuous flow microreactors can develop nanoparticles with a smaller mean particle size and narrower particle size distribution. The results showed that the mean particle diameter was decreased from 8.76 nm to 6.43 nm with the increase in flow rate from 15 to 35 mL/min 41. Fig. 4 shows the synthesis processes of nickel nanoparticles by continuous flow microreactor. Appalakutti et al., (2015) produced monodispersed copper chromite nanoparticles in a continuous flow micro channel reactor. Aqueous solutions of copper nitrate and chromium nitrate were used as the precursors. The particle size of obtained copper chromite nanoparticles was measured to be in the range 192–300 nm, relatively smaller compared to that obtained in a batch reactor 27.
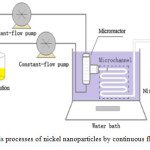 |
Figure 4: The synthesis processes of nickel nanoparticles by continuous flow microreactor 41 Click here to View figure |
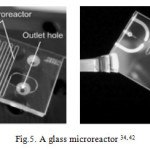 |
Figure 5: A glass microreactor 34, 42 Click here to View figure |
Spinning disc reactor (SDR)
Interest in spinning disc technology gradually evolved from the realization during the early 1900s that the flow of thin liquid films on plane surfaces due to gravity was of great practical importance in chemical engineering operations. Such films have been associated with surface wave formation, giving rise to intense mixing action within the film and increased heat- and mass-transfer rates 43. To achieve nanosized crystals from precipitation, high supersaturation levels that are uniform throughout the precipitation system are key processing requirements for high nucleation rates and small particles. The SDR has this capability 44.
Process intensification, using spinning disc processing (SDP), potentially offers an avenue for the production of monodisperse nanoparticles with controllable properties. The reagents are directed towards the center of the disc, which is rotated rapidly (300 and 3000 rpm) resulting in the generation of a very thin fluid film (1 to 200 μm). The thinness of the fluid layer and the large contact area between it and the disc surface facilitates very effective heat and mass transfer. The drag forces between the moving fluid layer and the disc surface enable very efficient and rapid mixing. The greatest strength of SDP synthesis is the broad range of control possible over all the operating parameters involved in nanoparticle formation, enabling the simultaneous and individual optimization of many interdependent operating mechanisms, with the ultimate goal of achieving very narrow particle size distributions 45. Chin et al., (2008) produced Fe3O4 nanoparticles at room temperature. These are formed by passing ammonia gas over a thin aqueous film of Fe 2+/ 3+ which is introduced through a jet feed close to the center of a rapidly rotating disc (500 to 2500 rpm), the particle size being controlled with a narrow size distribution over the range 5 nm to 10 nm 46.
Hartlieb (2010) in his thesis could produce Zinc oxide nanoparticles using SDP with a modified sol-gel technique. It that study, they found that SDP 3 parameters, such as disc speed, disc temperature, disc texture, and flow rate, influenced the rate of formation of zinc oxide nanoparticles and the size of the nanoparticles produced. Typically, faster disc speeds resulted in enhanced formation rates of zinc oxide as well as the formation of smaller particles, most likely due to the creation of micromixing conditions and the enhancement of homogeneous nucleation. Some limitations of spinning disc processing towards the formation of metal nanoparticles are: very short fluid residence times and metal deposition onto the surface of the disc. These limitations can be easily solved by increasing the disc diameter and modification of the material from which the disc is constructed 46. Raveendran et al., could synthesize silver particles with a mean size between 13 and 16 nm were formed in the SDR after 10 minutes of processing 47. Chin et al., synthesized super paramagnetic Fe3O4 nanoparticles at room temperature using continuous flow spinning disc processing (SDP). In their study Alginic acid, a natural biopolymer originating from algae, was employed as a surfactant to stabilize the Fe3O4 particles during processing in the SDR. The ability to inject the alginic acid into the magnetite particle suspension and achieve uniform mixing of the two streams on the rotating disc constituted a considerable processing advantage in producing highly stable particles of about 10 nm and of very narrow distributions 44. Mohammadi et al., (2014) produced titanium dioxide nanoparticles via a sol–gel route on a spinning disc reactor (SDR). They also studied the physical parameters such as rotational speed, disc surface texture, and operating parameters such as flowrate, ratio of water to precursor and location of feed introduction points in terms of their effects on TiO2 particle size, particle size distribution (PSD) and particle yield 48.
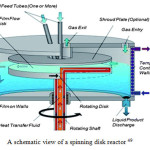 |
Figure 5 B Click here to View figure |
Conclusion
Since the wide range of Nano Particles applications in various fields and the need of using the intensification technology in order to minimize plant or equipment size in the synthesis process, reviewing some intensification techniques e.g., Segmented Flow Tubular Reactor, microreactors and spin disc reactor for synthesis process is the main aim of the present paper.
Process intensification refers to the intensification of production by increasing the rates of heat and mass transfer by orders of magnitude over conventional stirred batch reactors. Any chemical engineering development that leads to a substantially smaller, cleaner, safer and more energy efficient technology is process intensification 9. The implementation of process intensification techniques resulted in the improvement in green synthesis processes without damaging the environment and requiring less capital cost 7.
It was proved that the SFTR is a powerful tool for the production of powders and nanocrystals with advanced. In the past several years microreactors and microprocess engineering have been the subject of worldwide academic research. Microreactor technology has the ability to control the reactions at the micrometer scale. Synthesis in microreactors has several important advantages over conventional batch process synthesis. They include: (1) small volumes and short paths cause more uniform processing conditions and result in less byproduct formation, (2) chemicals that are dangerous to handle and stored in large quantities, can be produced safely using a microreactor as much as needed, (3) fast reactions which could accelerate uncontrollably in conventional reactors leading to possible explosions, can be run safely due to the small dimensions of the flow channels, and (4) better reaction kinetic data in the microreactor permitting better and faster optimization of conventional production reactors 34. Microreactor has also the ability to control the temperature of the reactor due to its high surface to volume ratio. The use of spinning disc processing (SDP) for the synthesis of nanomaterials is an attempt to overcome the problems associated with scaling-up production, achieve greater control of the size of the nanoparticles produced, and indeed gain access to new materials not possible using other processing techniques 46. It can be concluded that there is a need for more research works in this field in order to economic and large scale synthesis of nanomaterials.
References
- Ranjit,K., Baquee, A.A., Int. Res. J. Pharm. 2013, 4 (4),1-11. DOI: 10.7897/2230-8407.04408
- Hahens ,W.I., Oomen ,A.G., DeJong W.H., Cassee F.R., REGUL TOXICOL PHARM, 2007,49(3),217-229. http:// dx.doi.org/10.1016/j.yrtph.2007.07.006 PMid: 17868963
- Khodashenas, B., Ghorbani, H. R., International Journal of Nano Dimension, 2015,6(2), 111-127,.
- Sharma, V. K., Yngard, R. A., Lin, Y., Adv. Colloid Interfac, 2009,145 , 83-96.
- Mohanraj,V.J., Chen,Y., Tropical Journal of Pharmaceutical Research, 2006, 5 (1), 561-573.
- Wightman , E.P. , Trivelli , A.P.H. , Sheppard , S.E., 1927, J . Franklin Institute , 203(2) , 261-278. doi:10.1016/S0016-0032(27)92448-X
- Kumar , V., Nigam,K. D. P., Green Process Synth, 2012,1(1) , 79–107. DOI: 10.1515/greenps-2011-0003,
- Keller, G.E., Bryan, P.F., Chem. Eng. Progress, 2000, 41–50.
- Reay,D., Ramshaw, C., Harvey,A., 2013, Process Intensification (Second Edition) Engineering for Efficiency, Sustainability and Flexibility, A volume in Isotopes in Organic Chemistry , 27–55 doi:10.1016/B978-0-08-098304-2.00002-X
- BOODHOO, K., HARVEY, A., Edited by, School of Chemical Engineering & Advanced Materials Newcastle University, UK. This edition first published 2013. ISBN: 9780470972670
- Glaser, J.A., Clean Techn Environ Policy ,2012,14,155–160. DOI 10.1007/s10098-012-0466-5
- Adrian T., Schoenmakers H., Boll M., Chemical Engineering and Processing, 2004,43 (3), 347–355.
- Stankiewicz A.I., Moulijn, J. A., Chem. Eng. Prog., 2000,96(1), 22-34.
- Keil F.J., in : F.J. Keil (Ed.), Modeling of Process Intensification, WILEY-VCH Verlag GmbH & Co. KGaA, 2007.
- Sharma, V.K., Yngard, R.A., Lin, Y., Adv. Colloid Interface,2009, 145, 83–96.
- GHORBANI, H. R., PARSA MEHR ,F., KHANIYANI POOR ,A., ORIENTAL JOURNAL OF CHEMISTRY, 2015, 31 (1), 527-529. http://dx.doi.org/10.13005/ojc/310165
- HOSSEINI, S. J., AGHAIE, H. , GHAEDI, M. , ORIENTAL JOURNAL OF CHEMISTRY, 2014, 30(4), 1883-1895. http://dx.doi.org/10.13005/ojc/300449
- Khodashenas, B., Indian Chemical Engineer ,2015, in press. http://dx.doi.org/10.1080/00194506.2015.1026950.
- He, B.L., Tan, J.J., Kong ,Y.L., Liu, H.F., J. Mol. Catal. A Chem. 2004, 221, 121 – 126. doi:10.1016/j.molcata.2004.06.025
- Ramshaw ,C., Mass transfer process, European Patent No.0002568, 1979.
- Testino, A., Pilger,F., Alberto Lucchini , M., Quinsaat, J.E.Q., Stähli ,C., Bowen, P., Molecules ,2015, 20, 10566-10581. doi:10.3390/molecules200610566
- Aimable,A., Jongen,N., Testino,A., Donnet,M., Lemaître,J., Hofmann,H., Bowen, P., Chem. Eng. Technol., 2011,34(3), 344–352.
- Aimable, A., Strachowski, T., Wolska,E., Lojkowski, W., Bowen, P., Processing And Application Of Ceramics, 2010,4 (3) , 107–114.
- Dimian,A.C., Bildea, C.S., Kiss ,A.A., 2014,Integrated Design and Simulation of Chemical Processes, Second edition , Amsterdam : Elsevier Science.
- Lin,X., Terepka,A., Yang,H., Nano Lett. 2004,4 ,2227–2232.
- Patil,G., Bari,M., Bhanvase, B., Ganvir, V., Mishra,S., Sonawane,S., Chem. Eng. Process.: Process Intens. 2012,62,69–77.
- Appalakutti,S., Sonawane,S., Bhanvase,B.A., Mittal ,V., Ashokkumar, M., CHEM ENG PROCESS, 2015,89 ,28–34.
- Chung, C. K.; Shih, T. R.; Wu, B. H., in Transducers, Denver, CO, 2009.
- Gomez-de Pedro, S., Martinez-Cisneros, C. S., Puyol, M., Alonso-Chamarro, J., Lab. Chip., 2012, 12, 1979-1986.
- Khan, S. A.; Duraiswamy, S., Lab. Chip., 2012, 12, 1807-1812.
- Nightingale, A. M., Krishnadasan, S. H., Berhanu, D., Niu, X., Drury, C., McIntyre, R., Valsami-Jones, E., deMello, J. C., Lab. Chip., 2011, 11, 1221-1227.
- Marre, S., Adamo, A., Basak, S., Aymonier, C., Jensen, K. F., Ind. Eng. Chem. Res., 2010, 49, 11310-11320.
- Frenz, L., El Harrak, A., Pauly, M., Begin-Colin, S., Griffiths, A. D.; Baret, J. C., Angew. Chem. Int. Ed., 2008, 47, 6817-6820.
- Singh,A., Malek,C.K., Kulkarni, S.K., International Journal of Nanoscience, 2010,9(1 & 2), 93–112. DOI: 10.1142/S0219581X10006557
- Chan, E. M., Alivisatos, A. P., Mathies, R. A., J. Am. Chem. Soc., 2005,127, 13854-13861,.
- Abdelhady, A. L., Afzaal, M.; Malik, M. A.; O’Brien, P., J. Mater. Chem., 2011,21, 18768-18775.
- Wagner, J., Köhler, J.M. , Nano Lett. 2005, 5, 685–691.
- Singh,A., Shirolkar, M., Lalla,N.P., Malek, C.K., Kulkarni,S.K., International Journal of Nanotechnology ,2009,6 ,541–551.
- Wagner, J., Kirner, T., Mayer,G., Albert, J., Köhler, J.M., Chemical Engineering Journal, 2004,101,251–260.\
- Patil,G.A., Bari, M.L., Bhanvase, B.A., Ganvir , V., Mishra, S. Sonawane,S.H., Chemical Engineering and Processing, 2012,62 ,69– 77. http://dx.doi.org/10.1016/j.cep.2012.09.007
- Xu ,L., Srinivasakannan, C., Peng, J., Zhang,D., Chen, G., Chemical Engineering and Processing, 2015,93 ,44–49. http://dx.doi.org/10.1016/j.cep.2015.04.010
- Watts, P., Haswell, S. J., Chem. Eng. Technol., 2005,28(3), 290-301.
- Boodhoo, K. V. K., In Process Intensification for Green Chemistry; Boodhoo, K.; Harvey, A. P., Eds.; Wiley: Chichester, 2013. DOI: 10.1002/9781118498521
- Chin,S. F., Iyer,K. S., Raston C. L., Saunders,M., Adv. Funct. Mater., 2008,18, 922–927 .
- Chin,S.F., Iyer, K.S., Raston,C.L., Saunders,M., NSTI-Nanotech, 2008,1,782 – 785. ISBN 978-1-4200-8503-7
- Hartlieb, K., Process intensification and green chemistry strategies for the synthesis of nanomaterials, Thesis (Ph.D.)–University of Western Australia, 2010. Persistent URL : http://repository.uwa.edu.au:80/R/-?func=dbin-jump- full and amp ;object _id= 30387 & amp; silo_library=GEN01
- Raveendran, P. J. Fu , Wallen,S. L., J. Am. Chem. Soc., 2003, 125, 13940–13941 .
- Mohammadi,S., Harvey,A., Boodhoo,K.V.K., Chemical Engineering Journal, 2014, 258 ,171–184. http://dx.doi.org/10.1016/j.cej.2014.07.042
- Pask ,S.D., Nuyken,O., Cai,Z.Z., Polymer Chemistry, 2012, 3 ,2698–2707.

This work is licensed under a Creative Commons Attribution 4.0 International License.









