Thermal Stability, Sorption Properties and Morphology of Films of Dipeptide and Tripeptide Based on L-Glycine
Marat A. Ziganshin,1,*Sufia A. Ziganshina,1,2 Nadezhda S. Gubina,1 Alexander V. Gerasimov,1 Valery V. Gorbatchuk,1 and Anastas A. Bukharaev2
1A.M. Butlerov Institute of Chemistry, Kazan Federal University, Kremlevskaya18, Kazan, 420008 Russia. 2Kazan Zavoisky Physical-Technical Institute of the Kazan Scientific Center of the Russian Academy of Sciences, Sibirskiitrakt 10/7, Kazan, 420029 Russia. Corresponding Author Email: Marat.Ziganshin@kpfu.ru
DOI : http://dx.doi.org/10.13005/ojc/310415
Article Received on :
Article Accepted on :
Article Published : 21 Nov 2015
The effect of the number of amino acid residues in L-glycyl-L-glycine and L-glycyl-L-glycyl-L-glycine on thermal stability of powders, the sorption properties and surface morphology of thin films has been found. Dipeptide forms the film coated with disk-shaped nano-objects on the hydrophilic substrate, while tripeptide self-organizes to the film coated with nano-crystals on the hydrophobic substrate. Replacement of substrates (hydrophilic↔hydrophobic) leads to the formation of smooth films of studied oligopeptides. Powders of oligopeptides do not form stable clathrates with water and organic compounds at room temperature. But their thin films are capable to bind organic or water vapors with high thermodynamic activity. Surprising difference in sorption selectivity of dipeptide and tripeptide has been observed. L-Glycyl-L-glycine predominantly binds organic H-donors, while L-glycyl-L-glycyl-L-glycine is more selective to H-aceptors.
KEYWORDS:Oligopeptides; Morphology of film; Sorption; Thermal stability; Nanostructures
Download this article as:| Copy the following to cite this article: Ziganshin M. A, Ziganshina S. A, Gubina N. S, Gerasimov A. V, Gorbatchuk V. V, Bukharaev A. A. Thermal Stability, Sorption Properties and Morphology of Films of Dipeptide and Tripeptide Based on L-Glycine. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Ziganshin M. A, Ziganshina S. A, Gubina N. S, Gerasimov A. V, Gorbatchuk V. V, Bukharaev A. A. Thermal Stability, Sorption Properties and Morphology of Films of Dipeptide and Tripeptide Based on L-Glycine. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12672 |
Introduction
As a consequence of self-organization the short-chain oligopeptides are capable to form the materials based on well-arranged nanostructures, such asnanotubes1,2, nanofibers3, nanorods4,5, nanowires6,nanoparticles7, nanospheres5,8. Such materials are biocompatible9and used for the fabrication of sensitive sensors9,10, for preparation of hybrid materials11,12, as well as new sorbents, which capable to bind gases13,14, bioactive substances15and enantiomers16,17.Such possibilities of their practical usage in modern technologies caused the significant interests for oligopeptides in recent times18,19.
The study of self-organization of oligopeptides permitted us to establish that the form of nanostructures depends on type17,20, quantity21 and sequence22-24of amino acid residues in oligopeptide molecules.Also the form depends on the solvents used for crystallization of peptide materials25or for saturation of amorphous oligopeptide films26, from pH27and humidity28of environment, peptide concentration in solution29, and type of substrate used for creation of oligopeptide structure28,30.
Such variety of factors makes oligopeptides attractive for using as supramolecular structural blocks.However, on the other hand this feature complicates the development of techniques forcontrollable self-organization of oilgopeptides for obtaining of nanomaterials with preset structure and properties.This leads to the fact that normally researchers limit their study in relation to a small number of short-chain oligopeptides31, in particular diphenylamine8,32.
In the present work the effect of number of amino acid residues in L-glycyl-L-glycine (GG) and L-glycyl-L-glycyl-L-glycine (GGG) on morphology of surface of their films by atomic-force microscopy (AFM) was studied for the first time.Also the effect of quantity of amino acid residues on thermal stability of these oligopeptides was studied using thermogravimetric (TG) analysis with simultaneous differential scanning calorimetry (DSC) and mass-spectrometric (MS) detection of the evolved vapors. Their sorption properties towards vapors of water or organic compounds were investigated on a quartz crystal microbalance (QCM-sensor).
Experimental
Materials.Dipeptide L-glycyl-L-glycine (GG) (Chem-Impex, Cat# 001656)and tripeptideL-glycyl-L-glycyl-L-glycine (GGG) (Chem-Impex, Cat# 04555) were used without additional purification.
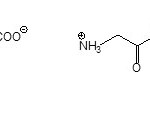 |
Scheme 1 Click here to View scheme |
Purified organic solvents33had at least 99.5% purity.
Thermoanalysis by Simultaneous TG/DSC/MS
Simultaneous thermogravimetry (TG) and differential scanning calorimetry (DSC) analysis of oligopeptide powder with mass spectrometric (MS) evolved gas analysis were performed using thermoanalyzer STA 449 C Jupiter (Netzsch) coupled with quadrupolar mass-spectrometer QMS 403C Aeolos (Netzsch) as described elsewhere34,35. In each experiment, the temperature rate was 10 K/min, and an argon atmosphere with a total flow rate of 75 ml/min was used. For this experiment, 5-7 mg samples of sorbate-free oligopeptide were placed in aluminum crucibles (40 μl) with lids having 3 holes, each of 0.5 mm in diameter. The samples of oligopeptide saturated with water or organic vapors were prepared in the same crucibles by equilibration of powder of oligopeptide with vapors of these sorbates (P/P0=1) for 72 h at 25°C in hermetically sealed 15 ml vials. The TG/DSC/MS experiment began after 20 minutes of sample equilibration at 25°C in argon flow of 75 ml/min. The sample mass loss was determined with the error of 5 %.
QCM study of sorbate binding
A sensor device with 10 MHz QCM crystals (Part No. 151620-10, ICM Co. Inc, USA) of thickness shear mode (TSM) was used34.The oligopeptide coatings (~0.80 μg) were prepared by drop and drying (for 2 min) by hot air (45°C) of methanol solution on the gold surface of quartz crystals.
In a QCM sensor experiment, a liquid sorbate was sampled using microsyringe to the sensor cell bottom through a dosing hole in the cell cover. The sampled sorbate amount was twice as large as necessary to create a saturation vapor in the sealed cell. Still, the cell was made not hermetical during sensor experiment to avoid sorbate condensation on the coating surface. The sorbaterelative vapor pressure P/P0was kept below saturation level by the vapor leak through the dosing hole. This level is equal to P/P0= 0.8536. A sensor baseline noise did not exceed 3 Hz. The frequency change of quartz crystal ∆F in sensor experiments was determined with the reproducibility of 5% for ∆F > 50 Hz.
To regenerate the oligopeptide coatings after the sorbatebinding, they were dried by hot air as described above. This regeneration procedure was repeated at least twice until the constant sensor frequency was achieved.
Atomic force microscopy (AFM)
AFM images in topography and phase modes were recorded using the atomic force microscope Solver P47 (NT-MDT, Russia)26,28. Measurements were performed in air using a tapping mode. Standard silicon cantilevers NSG-11 (NT-MDT, Russia) were used. For AFM experiments, oligopeptide films with diameter of 3 mm were prepared on the surfaces of highly oriented pyrolytic graphite (HOPG) or mica plates (5´5mm) using the same technique as for QCM study. HOPG and micawere freshly cleaved before use.
Results and Discussion
Thermal analysis of oligopeptides and its powders saturated with vapors of water and organic compounds
Thermalstability of powders of GG and GGG was studied using combined method of thermal analysis. Obtained results are given in Fig. 1.
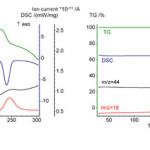 |
Figure 1: The data of TG/DSC/MS analysis for the powders of(a) GGand(b) GGG. Click here to View figure |
 
Dipeptide GG was found to melt above 230°С with loss of mass, Fig. 1a. TripeptideGGG melts at temperature 249°С, Fig. 1b. Mass loss of sample caused by its thermal decomposition was observed at temperature above 251°С. On mass-spectrometric curves there are signals of water (m/z=18) and carbon dioxide (m/z=44) above melting temperatures, Fig. 1. Higher thermal stability of GGG is apparently related to its molecular weight – 288 g/mol, as compared to 231 g/mol for dipeptide.
Below melting temperatures of studied oligopeptides on DSC curves there are no signals associated with its phase transition. Obtained results permit us to confirm that heating of thin films of oligopeptides up to 45°С in sensor experiment does not lead to change in its mass or chemical composition.
In the present work the products of oligopeptides saturated with vapors of organic compounds or water were studied. It was found that powder of GG and GGG do not form stable inclusion compounds neither with H-donors (water, methanol and hexafluoroisopropanol) nor H-acceptor (pyridine) at room temperature. On TG curves there are no signals associated with loss of mass. According to data of mass-spectrometric analysis in evolved vapors there are no sorbates used for saturation of oligopeptides. Examples of data of thermal analysis of powders of GG and GGG after saturation with vapors are given in Fig. 2.
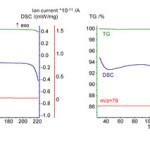 |
Figure 2: The data of TG/DSC/MS analysis for theproducts of oligopeptides (a) GG and (b) GGG saturation with vapor of pyridine, P/P0=0.85, T=298K. Click here to View figure |
For further study of interaction of oligopeptides GG and GGG with vapors the sensor analysis was used. This method allows to study heterogenic systems “solid/vapor” with thermodynamic activity of vapors P/P0=0.85,while in thermal analysis the thermodynamic activity of vapors P/P0 is near to zero.
The sensor analysis of vapors sorption by GG and GGG
We have determined the composition of saturation products of thin films of oligopeptides of GG and GGG with vapors using the quartz crystal microbalances. Examples of sensor responses are shown in Fig. 3.
The calculated sorbate/oligopeptide molar ratios S from sensor data are given in Table 1. In Table 1 is also given the values of molar refraction MRD of studied sorbents. MRD is a good parameter to describe molecular size22,37.
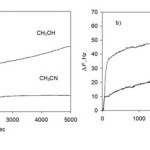 |
Figure 3: Responses of QCM sensor coated with films of (a) GG and (b) GGG to organic vapors with relative vapor pressure P/P0 = 0.85 at T = 298 K. Sensor responses ΔF are normalized to the coating mass with corresponding frequency decrease of ΔFdipeptide = 1000 Hz. Click here to View figure |
Table 1: The sorbate/oligopeptide molar ratio calculated from QCM sensor data.a
|
Sorbate |
MRD,b cm3/mol |
S, molsorbate / molGG |
S, molsorbate/ molGGG |
|
|
1 |
Н2О |
3.7 |
0.56 |
0.44 |
|
2 |
CH3OH |
8.2 |
0.54 |
0.15 |
|
3 |
CH3CN |
11.1 |
0.10 |
0.24 |
|
4 |
CH3NО2 |
12.5 |
0.03 |
0.18 |
|
5 |
C2H5OH |
13.0 |
0.13 |
0.06 |
|
6 |
C2H5CN |
16.0 |
0.08 |
0.16 |
|
7 |
СНCl3 |
21.3 |
0.08 |
0.13 |
aSorption of pyridine vapor does not exceed of experimental error; bMRD =(M/d)´(nD2 – 1)/(nD2 + 2), where M is molecular weight of sorbate, d and nD are density and refractive index of liquid sorbate, respectively
It was found that GG has a maximum sorption capacity to water and methanol, GGG – to water, Table 1. Increase of size of sorbate molecules leads to decrease of sorption capacity of studied oligopeptides. For example, from methanol to water the molecule size increases at 2.2 times and sorption capacity of GG decreases to about 4%, while for GGG this value decreases to ~ 66%.
For analysis of effect of quantity of amino acid residues in GG and GGG on their sorption properties the correlation between the sorption capacity of GGG and GG was plotted, Fig.4. Point numbers correspond to the sorbent numbers from Table 1.
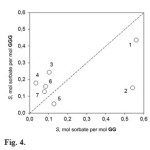 |
Figure 4: Correlation between sorbate/oligopeptide molar ratios S of saturated products of GG and GGG. Point numbers correspond to the sorbatenumbers in Table 1. Click here to View figure |
It was found that dipeptide has selectivity for H-donors (alcohols) and water, while tripeptide is selective to H-acceptors (nitriles), Fig. 4. Probably this is caused by different packing of oligopeptide molecules on hydrophobic surface of gold electrode. In case of dipeptide the presence of free acceptor groups in thin layer capable to interact with H-donors may be assumed. Whereas in the film of tripeptide there are free H-donor groups.
Morphology of thin films of oligopeptides according to AFM data
To study the morphology of surface of thin films of oligopeptides the images of films deposited on highly oriented pyrolytic graphite (HOPG) or mica were obtained, Fig. 5-7.
The surface of HOPG as well as gold coating of quartz crystal microbalances used in present work is hydrophobic. In contrast to HOPG the surface of mica is hydrophilic and may be charged.
AFM data obtained in present work permitted us to enquire the state of thin films on surface of quartz crystal microbalances. Also the effects of substrate and number of amino acid residues in oligopeptide on morphology of its film were studied.
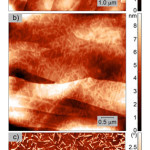 |
Figure 5: AFM images of the surface of the films deposited on HOPG from a methanol solution:in topography mode (a) GG, (b) GGG and in phase contrast mode (c) GGG. Click here to View figure |
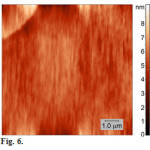 |
Figure 6: AFM images of the surface of the GGG film deposited on mica from a methanol solution (topography mode). Click here to View figure |
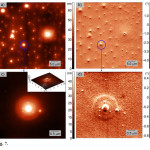 |
Figure 7: AFM images of the surface of the GG film deposited on mica from a methanol solution: in topography mode (a, c) and in phase contrast mode (b, d). The inset shows the 3D image of an object from Fig. 7c. Click here to View figure |
It was found that dipeptide GG forms the smooth film on HOPG, Fig.5a. The average height spread on a 10×10 µm scan does not exceed 10 nm. A mean square roughness of the surface (Rq) was 0.96±0.05. Beneath the surface of film the crystallographic steps of graphite are clearly visible. Whereas tripeptideGGG forms the film uniformly covered by complex crystallographic objects with width 50-100 nm, length 300-700 nm, and height 0.4-2 nm, Fig. 5b,c. The average height spread on a 5×5 µm scan was 9 nm. The crystallographic steps of HOPG do not influence on distribution and shape of crystals of GGG.It is to be noted that some crystalline objects on film surface intersect. The intersection angles are 50-70° (60° is more often).
Earlier we have found that dipeptides L-alanyl-L-valine and L-valyl-L-alanine also form the smooth amorphous films on HOPG surface22. While on surface of the films of L-leucyl-L-leucine28 and L-leucyl-L-leucyl-L-leucine26 there are spherical objects and crystals, correspondingly.
On the surface of mica tripeptideGGG forms smooth film, Fig. 6. The average height spread on a 10×10 µm scan does not exceed 10 nm.A mean square roughness of the surface (Rq) was 0.34±0.02.
On the surface of mica GG self-organizes into film uniformly covered by objects of spherical form with a base diameter from 200 to 3000 nm, Fig. 7a,b. The average height spread on a 50×50 µm scan was 70 nm. The large objects have a complicated form reminding pyramid, which built of a few discs of different diameter. Example of such object is given in Fig. 7c,d. Thickness of discs of pyramids vary from 10 to 25 nm, Fig.7. On top of the pyramids there are ledges with height from 17 to 35 nm, base diameter of 700-1000 nm and diameter at peak of 200 nm. The small spherical objects with height of 25-40 nm have close diameter at top and base diameter from 1.4 to 2.5 µm. It is to be noted that such objects earlier were not described in the literature.
So, AFM study of films of GG and GGG shows that depending on the type of substrate (hydrophilic or hydrophobic) the films with different morphology may be obtained. Dipeptide forms a smooth film on hydrophobic surface, while the tripeptide forms such film on hydrophilic surface.
Conclusions
In the present work the thermal stability of powders of L-glycyl-L-glycine and L-glycyl-L-glycyl-L-glycine and its receptor properties toward vapors were studied. It was found that tripeptide has higher thermal stability compared to dipeptide. The sorption capacity of studied oligopeptides generally decreases with increase of molecule size of sorbates. Herewith dipeptide and tripeptide show the selectivity to H-donor and H-acceptor compounds, correspondingly. The morphology of thin films of L-glycyl-L-glycine and L-glycyl-L-glycyl-L-glycine on different substrates was studied.It was observed that dipeptide forms a film covered with spherical objects on hydrophilic surface of mica and smooth film on hydrophobic surface of HOPG. In case of tripeptide a smooth film formed on mica surface, but on hydrophobic surface of HOPG film is covered with crystalline objects. Obtained results may be useful for design of organic films with necessary morphology.
Acknowledgements
This study was supported by Russian GovernmentProgram of Competitive Growth of Kazan Federal University.
References
- Hamley, W. Angew. Chem., Int. Ed., 2014, 53, 6866-6881.
- Wang, M.;Du, L.;Wu, X.;XiongS.; Chu, P. K. ACS Nano, 2011, 5, 4448-4454.
- Mahler, A.;Reches, M.;Rechter, M.;Cohen S.;Gazit, E. Adv. Mater., 2006, 18, 1365-1370.
- Ryu,J.;Park, C. B. Chem. Mater., 2008, 20, 4284-4290.
- Guo, C.;Luo, Y.; Zhou R.;Wei, G. Nanoscale, 2014, 6, 2800-2811.
- Ryu,J.;Park, C. B. Adv. Mater., 2008, 20, 3754-3758.
- Sun, L.;Fan, Z.;Wang, Y.; Huang, Y.;Schmidt M.;Zhang, M. Soft Matter, 2015, 11, 3822-3832.
- Sakurai, M.;KoleyP.;Aono, M. Chem. Commun., 2014, 50, 12556-12559.
- Yan, X.; Zhu, P.; Li, J. ChemSoc Rev. 2010, 39, 1877-1890.
- Kim, J. H.; Ryu,J.; Park C. B., Small, 2011, 7, 718-722.
- Ryu, J.; Kim, S-W.; Kang, K.; Park, C. B. Adv Mater.,2010, 22, 5537-5541.
- Zhao, X.; Pan, F.;Xu, H.;Yaseen, M.; Shan, H.; Hauser, C.A.E.; Zhang, Sh.; Lu, J.R. Chem. Soc. Rev.2010, 39, 3480-3498.
- Afonso, R. V.;Durão, J.; Mendes, A.; Damas,A. M.;GalesL.Angew. Chem., Int. Ed., 2010, 49, 3034-3036.
- Comotti, A.;Fraccarollo, A.;Bracco, S.;Beretta, M.;Distefano, G.;Cossi, M.;Marchese, L.;Riccardi,C.;Sozzani,P.CrystEngComm, 2013, 15, 1503-1507.
- Burchell, T.J.;Soldatov, D.V.;Ripmeester, J.A. J.Struct. Chem. 2008, 49, 188-191.
- Akazome, M.; Ueno, Y.;Ooiso, H.; Ogura, K. J. Org. Chem.,2000, 65, 68-76.
- Gorbitz, C. H. Chem. Eur. J.,2007, 13, 1022-1031.
- Gill, J. P. K.; Singh, S.; Sethi, N. Orient. J. Chem., 2015, 3, 417-42.
- Balali, E. Orient. J. Chem., 2014, 30, 623-629.
- Haldar, D.;Maji, S.K.;Sheldrick, W. S.; Banerjee, A.Tetrahedron Letters,2002,43, 2653-2656.
- Tamamis, P.;Abramovich, L. A.;Reches, M.; Marshall, K.;Sikorski, P.;Serpell, L.;GazitE.;Archontis, G.Biophys. J.,2009, 96, 5020-5029.
- Ziganshin, M.A.;Gubina, N.S.;Gerasimov, A.V.; Gorbatchuk, V.V.;Ziganshina, S.A.;Chuklanov,A.P.;Bukharaev,A.A.Phys. Chem. Chem. Phys., 2015, 17, 20168-20177.
- Dutta, A.;Dutt, A.; Drew, M.G.B.; Pramanik, A. Supramolecular Chemistry,2008,20, 625-633.
- Joshi, K.B.;Verma, S.J. Pept. Sci.,2008,14, 118-126.
- Ariga, K.; Kikuchi, J.;Naito,M.; Koyama,E.; Yamada, N. Langmuir, 2000, 16, 4929-4939.
- Ziganshin, M. A.;Efimova, I. G.;Gorbatchuk, V. V.;Ziganshina, S. A.;Chuklanov, A. P.;Bukharaev,A. A.;Soldatov, D. V. J. Pept. Sci., 2012, 18, 209-214.
- Matsui, H.; Holtman, C. NanoLett.,2002,2, 887-889.
- Ziganshin, M. A.;Bikmukhametova, A. A.;Gerasimov, A. V.;Gorbatchuk, V. V.;Ziganshina, S. A.;Bukharaev,A. A. Prot. Met. Phys. Chem. Surf., 2014, 50, 49-54.
- Yan, X.; Cui, Y.; He, Q.;Wang, K.; Li, J.; Mu, W.; Wang, B.;Ou-Yang, Z. C. Chem. Eur. J.,2008,14, 5974-1580.
- Ziganshin, M. A.;Efimova, I. G.; Bikmukhametova, A. A.;Gorbachuk, V. V.;Ziganshina, S. A.;Chuklanov, A. P.;Bukharaev,A. A. Prot. Met. Phys. Chem. Surf., 2013, 49, 274-279.
- Panda,J. J.;Chauhan,V. S.Polym. Chem., 2014, 5, 4418-4436.
- Rymer, S.-J.;Tendler, S. J. B.;Bosquillon, C.;Washington C.; RobertsC. J.Therapeutic Delivery,2011,2, 1043-1056.
- ArmaregoW. L. F. andChaiC. L. L., Purification of laboratory chemicals; Oxford, Butterworth-Heinemann. UK, (2009).
- Yakimova, L. S.;Ziganshin, M. A.;Sidorov, V. A.;Kovalev, V. V.;Shokova, E. A.;Tafeenko,V. A.;Gorbatchuk, V. V. J. Phys. Chem. B, 2008, 112, 15569-15575.
- Gataullina, K. V.;Ziganshin, M. A.;Stoikov, I. I.;Gubaidullin,A. T.;Gorbatchuk, V. V. Phys. Chem. Chem. Phys., 2015, 17, 15887-15895.
- Safina, G. D.;Validova, L. R.;Ziganshin, M. A.;Stoikov,I. I.;Antipin,I. S.;Gorbatchuk, V. V.Sens. Actuators B, 2010, 148, 264-268.
- Ziganshin, M. A.;Gerasimov, A. V.;Gorbatchuk,V. V.;Gubaidullin, A. T. J. Therm. Anal. Calorim.,2015, 119, 1811-1816.

This work is licensed under a Creative Commons Attribution 4.0 International License.









