Synthesis of Some Functionalized Ionic Liquids with Long Chain of Carbone Starting from Imidazole
Ahmed Djellal and Samia Amirat
Department of chemistry, Faculty of Sciences, University of Annaba, BP 12, Annaba -23000- Algeria. Corresponding Author Email: adjellaldz@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/310469
Article Received on :
Article Accepted on :
Article Published : 02 Dec 2015
Synthesis of some ionic liquids ILs 3 a-b is described starting from imidazole 1. The starting material is converted under microwave irradiation to N-alkyl imidazole (1a-b) which reacted with allylbromide to give functionalized N-alkyl, N’-allyl imidazolium bromide (2a-b). Hydroboration followed by methanolysis and acid hydrolysis applied to these give the target compounds 3 a-b in good yields.
KEYWORDS:Ionic liquid; imidazole; microwave irradiation; allylbromide; hydroboration
Download this article as:| Copy the following to cite this article: Djellal A, Amirat S. Synthesis of Some Functionalized Ionic Liquids with Long Chain of Carbone Starting from Imidazole. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Djellal A, Amirat S. Synthesis of Some Functionalized Ionic Liquids with Long Chain of Carbone Starting from Imidazole. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12785 |
Introduction
Ionic liquids (ILs), made of relatively large organic cations and inorganic anions, could contribute as solvents and catalysts to green organic synthetic reactions1-4. It was found that increasing the chain length of alkyl substituent on both cations and anions leads to greater lipophilicity of the ionic liquid 5, 6. The purpose of this investigation was the synthesis of functionalized ionic liquids with long chain of carbon in order to increase their lipophilicity starting from imidazole. The advantages of our synthesized ILs are: their high lipophilicity, their complexing power with e.g carbohydrates or alcohols and their propriety as phase transfer catalytic agent respectively provided by the presence of long chain of carbon, the boronic function and the imidazolium nucleus (Fig.1).
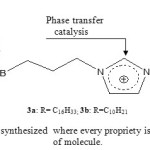 |
Figure 1: Ionic liquids synthesized where every propriety is assigned to each branch of molecule. Click here to View figure |
 
Step 1: Synthesis of N- alkyl imidazole 1a-b
We first used classical method CM9 to synthesize 1 a-b in order to have some reference for TLC control. Imidazole 1 was refluxed with alkyl halides in toluene for 3 h and left overnight under stirring at room temperature providing 1 a-b in 80% yield. We next wanted to transpose this reaction under microwave heating and establish optimal conditions of reaction (Scheme 1). We decided to try two types of microwave control : the first type is the classical heating with the control temperature/time (the microwaves control the irradiation power to maintain fixed temperature ). The second is the power/time control with the infrared measurement of the temperature reached in the mixture. The first type11 is convenient and the reaction was performed as following :Imidazole 1 with 50% excess alkyl bromide and a catalytic amount of tetrabutylammonium bromide (TBAB) was adsorbed on the mixture of potassium carbonate and potassium hydroxide ratio 1:1 and then irradiated at 50°C in an open vessel in a domestic microwave oven for 5 min till it changed to reddish orange color to give N-alkylimidazole 1 a- b in 90% yield. We compared classical method CM with microwave method MW and the results are summarized in table 1.
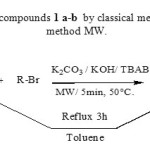 |
Scheme 1 : Synthesis of compounds 1 a-b by classical method CM and microwave method MW. Click here to View scheme |
Table 1: Reaction yields for the synthesis of N-alkylimidazole using CM and MW methods.
| Entry R- X N-alkylimmidazole Yield (%) (first step)CM MW |
| 1 C16H33-Br 1a 80 902 C10H21-Br 1b 82 94 |
Step2 : Synthesis of N-alkyl, N’-allyl imidazoliumbromide 2a-b:
Allylbromide refluxed with 1a-b in free solvent condition11 leadsto 2 a-b
(Scheme 2). There in 1H NMR a deshielding of methylene allylic protons group (4 to 5 ppm) proving that N’-allylation reaction was well conducted.
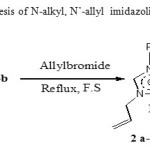 |
Scheme 2: Synthesis of N-alkyl, N’-allyl imidazoliumbromide 2a-b Click here to View scheme |
Step 3 : Synthesis of ILs 3 a-b
Hydroboration12 in standard conditions of 2 a-b followed by methanolysis and acid hydrolysis affords to the desired ILs 3a-b in 85% yield (scheme 3). H.C. Brown12 proposed acid hydrolysis of boranes to achieve the corresponding boronic acids. Because our non isolated intermediate boranes are immiscible in aqueous phase, 5 ml
of methanol was added drop wise to the mixture until it became homogeneous followed by hydrolysis with 5 ml of 1M hydrochloric acid. In 1HNMR we note the absence of peaks due to the ethylene protons group (between 5 and 6 ppm) proving the reduction of the allylic double bond. Note that we have used a small excess of BH3 in order to consume compounds 2 a-b.
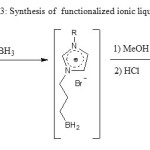 |
Scheme 3 |
Experimental
The 1H NMR spectra and 13C NMR were recorded in CDCl 3 using a spectrometer BRUKER AC 250 Fourier Transform (250 MHz). The chemical shifts are expressed in parts per million (ppm) relative to tetramethylsilane (TMS). The transfer of different sugars is followed by Waters 600 HPLC coupled with UV detector Waters 480. The data are processed using an integrator Shimadzu C-R4A.
In a wide-necked Erlenmeyer flask are introduced 6.8g (0.1 mol) imidazole, 45.5g (0.1 mol) 1-bromohexadecan, 2.4g (7.5mmol) tertiobutyllammoniumbromid (TBAB). The mixture is adsorbed on mixture of potassium carbonate and potassium hydroxide ratio 1:1 and then irradiated in an open vessel in a domestic microwave oven 300 Watt power for 3 min by period of 20 seconds till it changed to pasty.
After dilution in dichloromethane followed by washing with water, the organic phase is separated and dried over sodium sulfate. After filtration, the solvent is evaporated under vacuum.
1-hexadecyl-imidazole (1a)
Yield: 80%, yellow solid slightly pasty, 1H NMR (CDCl 3) δ ppm: 0.8 (t, 3H, CH3), 1.2 (m, 26H, carbon chain), 1.7 (m, 2H, N-CH2-CH2), 3.87 (t, 2H, N-CH2), 6.8 (s, 1H, H imidazole), 7.0 (s, 1H, H imidazole), 7.4 (s, 1H, H imidazole).
1-decyl-imidazole (1b)
Yield: 90%. yellow solid pasty, 1H NMR (CDCl 3) δ ppm: 0.8 (t, 3H, CH3), 1.2 (m, 14H, carbon chain), 1.7 (m, 2H, N-CH2-CH2), 3.87 (t, 2H, N-CH2), 6.8 (s, 1H, H imidazole), 7.0 (s, 1H, H imidazole), 7.4 (s, 1H, H imidazole).
Representative procedure for preparation of N-alkyl, N’-allyl imidazoliu bromide 2a and 2b
In a necked 50 ml equipped with a condenser, 4.9 ml (57 mmol) of allyl bromide are added dropwise to 0.01 mol of 1a (2.92g) or 1b (2.08g) in room temperature and free solvent conditions. The reaction mixture is refluxed and stirred until a solid slightly pasty appears. This residue is triturated with ether and then filtered under vacuum to obtain a solid dough.
1-hexadecyl-3-allyl-imidazolium bromide(2a)
Yield: 79%, pasty yellow solid; 1H NMR δ ppm 0.8 ppm (t, 3H, CH3), 1.2 ppm (m, 26H, carbon chain), 1.8 ppm (m, 2H, N-CH2-CH2), 4.3 ppm (t, 2H, N-CH2-C15H31), 5.0 ppm (d, 2H, CH2 = CH-CH2-N), 5.3 ppm (d, 1H, H allyl), 5.4 ppm (d, 1H, H allyl), 5.9 ppm (m, 1H, H allyl), 7.5 ppm (s, 2H, 2H imidazolium), 10.3 ppm (s, 1H, imidazolium H).
1-decyl-3-allyl-imidazolium bromide (2b)
Yield: 86% Appearance: pasty yellow solid, 1 H NMR (CDCl 3) δ ppm: 0.8 (t, 3H, CH3), 1.2 (m, 14H, carbon chain), 1.8 (m, 2H, N-CH2-CH2), 4.2 (t, 2H, N-CH2-C9H19), 5.0 (d, 2H, CH2 = CH-CH2-N), 5.3 (d, 1H, H allyl), 5.4 (d, 1H, H allyl), 5.9 ( m, 1H, H allyl), 7.49 ppm (s, 1H, imidazolium H), 7.52 ppm (s, 1H, imidazolium H), 10.3 (s, 1H, imidazolium H).
Synthesis of ILs 3a and 3b
In a three-necked flask fitted with a condenser and under argon, 10 mmol of N-alkyl, N’-allylimidazole are added to 50 ml of chloroform in an ice bath. We added 1.15 ml (12 mmol) of BH3 borane dimethyl sulfide (BMS). Then allowed to stir at room temperature for 3 hours. 5 ml of methanol are added dropwise to the reaction mixture before hydrolysis with 5 ml of 1M hydrochloric acid. The aqueous phase is washed with chloroform. The organic phases are combined, dried over sulfate and evaporated under
3-(3-hexadecylimidazolium) propyl boronic acid bromide (3a)
Yield: 81% Appearance: orange yellow resinous solid, 1H NMR (CDCl 3) δ ppm: 0.8 (t, 3H, CH3), 0.9 (m, 2H, (OH) 2B-CH2), 1.2 (m, 26H, chain carbon), 1.9 (m, 2H, N-CH2-CH2-C14H29), 1.9 (m, 2H, (OH) 2B-CH2-CH2-CH2-N), 4.2 (m, 2H, (OH) 2B-CH2 -CH2-CH2-N), 4.2 (t, 2H, N-CH2-C15H 31), 7.4 (s, 2H, 2H imidazolium), 10.3 (s, 1H, imidazolium H). 13C RMN (CDCl 3) δ ppm: 14 (CH3) 19 ((OH) 2B-CH2-CH2-CH2-N), 23 (CH2-CH3), 26 (OH) 2B-CH2-CH2-CH2-N), 28-30 (N-C13H26-CH2-CH2-CH3), 49 (N-CH2-C15H31), 50 ((OH) 2B-CH2-CH2-CH2-N), 122 (2C imidazolium), 136ppm (1C imidazolium ).
3-(3-decylimidazolium) propyl boronic acid bromide (3b)
M = 374.8 g / mol, Yield: 85% Appearance: Yellow viscous oil-orange, 1H NMR (CDCl 3) δ ppm: 0.8 (t, 3H, CH3), 0.9 (m, 2H, ( OH) 2B-CH2), 1.2 (m, 14H, carbon chain), 1.9 (m, 2H, N-CH2-C8H17), 1.9 (m, 2H, (OH) 2B-CH2-CH2 -CH2-N), 4.2 (m, 2H, (OH) 2B-CH2-CH2-CH2-N), 4.2 (t, 2H, N-CH2-C9H19), 7.4 (s, 2H, 2H imidazolium), 10.3 (s, 1H, H imidazoulium). 13C NMR (CDCl 3) δ ppm: 14 (CH3), 19 ((OH) 2B-CH2-CH2-CH2-N), 23 (CH2-CH3), 26 (OH) 2-B-CH2-CH2-CH2 – N), 28-30 (N-CH2-C7H14-CH2-CH3), 49 (N-CH2-C9H19), 50 ((OH) 2-B-CH2-CH2-CH2-N), 122 (1C imidazolium), 136 (imidazolium 2C).
Conclusion
We have synthesized some functionalized acid ionic liquids starting from imidazole with good yield using a microwave irradiation avoiding the long time of reaction and the risk of degradation of our products compared to the conventional method .
References
- Lancaster, M.; Green Chemistry: An Introductory Text, Roy. Soc. Chem., Cambridg, 2002, 154–161.
- (a) Murugesan, S.; Linhardt,R. J. Current Organic Synthesis, 2005, 2, 439- 440. (b) Zhao, B. Greiner, L.; Leitner, W.; RSC Adv. 2012, 2, 2476-2479.
- Lucinda J. A. Conceiçao, Ewa Bogel-Łukasik and Rafał Bogel- Łukasik, RSC Adv., 2012, 2, 1846-1855.
- Małgorzata Ewa Zakrzewska, and Rafał Bogel- Łukasik Energy Fuels 2010, 24 (2), pp 737–745.
- (a) Welton, T.; Chem. Rev.1999, 2073. (b) Hallett, J. P.; Welton, T.;Chem. Rev. 2011, 111, 3508.
- Muthusamy, S. ; Gnanaprakasam, B. ; Tetrahedron Lett., 46, 2005. 635.
- These compounds are recommended for many applications such as catalyst, inhibitor agent in corrosion, lubrification and other. Loupy, A. Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, 2002.
- Tierney, J.; Lindstrom, P. Microwave Assisted Organic Synthesis; Blackwell, London, 2005.
- Bogdal, D.; Pielichowskin J. Jaskot, K.; Heterocycles, 45,1997, 715-722.
- R. S. Varma and V. V. Namboodiri, Pure and Applied Chemistry, Vol. 73, No. 8, 2001, pp. 1309-1313.
- Buck, J.S.; Ferry, C. W.; Org. Syn. Coll. Vol. 2,1943, 290.
- Brown, H. C.; Organic Synthesis via Boranes. Ed John Wiley & Sons 1975.

This work is licensed under a Creative Commons Attribution 4.0 International License.









