Structural studies of UHMWPE composite materials containing nanoparticles of titanium, zirconium and hafnium oxides
A. M. Nemeryuk and M. M. Lylina
Federal State Unitary Enterprise ”State Research Institute of Chemical Reagents and Pure Chemical Substances”(FSUE “IREA”), 107076, Russia, Moscow, Bogorodskiy val, 3
DOI : http://dx.doi.org/10.13005/ojc/310484
Article Received on :
Article Accepted on :
Article Published : 10 Dec 2015
Studies have been conducted on composite materials based on UHMWPE containing nanoparticles of titanium, zirconium and hafnium oxides by DSC, DRS, IR, XRF and SEM.
KEYWORDS:UHMWPE; composite materials; nanoparticles; DSC; DRS; IR; XRF; SEM; TiO2; ZrO2; НfO2
Download this article as:| Copy the following to cite this article: Nemeryuk A. M, Lylina M. M. Structural studies of UHMWPE composite materials containing nanoparticles of titanium, zirconium and hafnium oxides. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Nemeryuk A. M, Lylina M. M. Structural studies of UHMWPE composite materials containing nanoparticles of titanium, zirconium and hafnium oxides. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13216 |
Introduction
One of the most promising structural materials – ultra-high molecular weight polyethylene (UHMWPE), which includes high-density polyethylene (HDPE) with a molecular weight of more than 106. It has a unique set of physical, mechanical and chemical properties, abrasion resistance, crack resistance and impact resistance, frost resistance , low coefficient of friction, as well as the ability to retain properties over a wide temperature range, so that demand in many industries [1].
One method of preparation of UHMWPE products, including fibers and films used to manufacture fabrics having high mechanical properties and are used for manufacturing products capable of withstanding the extremely strong shock and explosive loading is a method of processing UHMWPE via intermediate preparation of gels UHMWPE and subsequent stretching the filaments or films from such gels, ending with removal of the solvent used to prepare the gel [2].
Requirements for the construction and special materials that best meet the needs of modern technology (primarily aerospace) stimulated the development and widespread use of composite materials (CM), especially polymeric composite materials (PCM). In foreign science and technology to composite materials (Composite Materials) often include materials used as filler continuous high modulus fibers (boron, carbon, SiC, UHMWPE, Kevlar), textile form of them (filament bundles, ribbons), allowing design structure of composite materials to provide optimal properties for different types of loading [3].
We investigated composite materials based on UHMWPE containing metal oxides such as zirconium, titanium, hafnium prepared using processing methods similar to practically applied in the production of UHMWPE, such as obtaining UHMWPE gels. Introduction of the filler material based on the UHMWPE occurred by direct preparation of ultrafine or nanosized filler particles of oxide directly in the gels prepared from UHMWPE.
Methods
Obtained during the work-based composite materials UHMWPE containing nanoparticles of oxides of titanium, zirconium and hafnium investigated by various methods, including differential scanning calorimetry (DSC), dielectric relaxation spectroscopy (DRS), infrared spectroscopy (IR), X-ray fluorescence analysis (XRF) and scanning electron microscopy (SEM). These methods allow to obtain data about the material, showing its structure and properties, including the presence or absence of phase transitions in the temperature range covered by operating range devices used for studies [4]. Direct and indirect evidence, giving an idea of the composition and structure of materials allow to identify patterns associated with the peculiarities of the influence of the conditions for obtaining composite nanomaterials on their morphological characteristics and chemical composition.
Results and Discussion
In the course of investigations by DSC revealed phase transitions in materials based on UHMWPE containing nanoparticles of transition metal oxides, not peculiar to the starting UHMWPE. Thus is confirmed the previously expressed position on the impact of nanosized additives on the supramolecular structure of UHMWPE, reflected in the increase in the degree of crystallinity, which has a beneficial effect on the properties of materials, as most of the ordering of packaging macromolecules polymer promotes the values of the strength, abrasion resistance [5] . Of particular note is the growth temperature of the onset of melting materials based on UHMWPE containing nanoparticles of oxides of zirconium and hafnium, and a more pronounced influence of hafnium oxide nanoparticles that can be attributed to a greater polarization of the surface of these particles.
Table 1 summarizes the data obtained during the study of samples based nanomaterials UHMWPE containing nanoparticles of titanium oxides, zirconium and hafnium by DSC.
Table 1: Data materials research based on UHMWPE by DSC
|
Name of the sample |
Phase transition temperature 1, оС |
Phase transition temperature 2, оС |
Phase transition temperature 3, оС |
The temperature of the onset of melting, оС |
| UHMWPE |
no |
no |
no |
110 |
| UHMWPE + TiO20,1 % |
76 |
89 |
98 |
112 |
| UHMWPE + ZrO20,1% |
no |
91 |
100 |
114 |
| UHMWPE + HfO20,1% |
no |
93 |
101 |
116 |
According to Table 1, and materials containing oxides of zirconium and hafnium, a phase transition at 91-93oS is absent in the starting UHMWPE. Also an increase in the onset temperature of melting at the transition from the oxides of titanium to oxides of hafnium, with an equal percentage, indicating the greater effect of hafnium oxide on the crystallinity of UHMWPE.
In the study of the substance by the DRS determined the dielectric loss tangent at certain temperatures. UHMWPE samples containing the metal oxides as well as starting UHMWPE were investigated by RSM in the temperature range from – 170°C to 150°C.
DRS spectra of the original UHMWPE and modified UHMWPE material oxides have similar features, but the dielectric loss tangent at temperatures
from – 80°C to – 50°C is more important, reaching 1*10-5 for samples containing hafnium oxide, somewhat smaller value of the angle of dielectric losses are determined for sample with the nanoparticles of titanium oxide and zirconium, whereas the starting UHMWPE dielectric loss tangent within this temperature range an order of magnitude less.
Similar values of dielectric loss tangent obtained at temperatures ranging from 60°C to 90°C. At the moment the theoretical basis of the data is a subject of study, but the increase of the scattering of electromagnetic energy in the medium UHMWPE samples containing oxides of titanium, zirconium and hafnium, indicates a greater polarizability of the medium, which is associated with the presence of oxide particles.
Also, the dependence of the dielectric loss tangent of the chemical nature of the filler, in the presence of hafnium oxide value of the dielectric loss tangent is higher than in the presence of oxides of zirconium and titanium, which is correlated with the data of DSC, demonstrating a greater impact on the hafnium oxide supramolecular polymer packaging. The latter relates to the degree of polarization of the surface of the filler particles. Thus, the data obtained by RSM confirm a theoretical model relating the degree of polarization of the surface of the filler particles with the influence on the properties of the polymer matrix.
Studies by IR spectroscopy was used to confirm the presence of benzyl alcohol in the polymeric matrix UHMWPE synthesized as an intermediate in the process and to obtain samples of UHMWPE modified nanoparticles of titanium oxides, zirconium and hafnium. Figures 1 and 2 show the IR – spectra of the starting UHMWPE and UHMWPE containing benzyl alcohol.
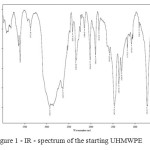 |
Figure 1: IR – spectrum of the starting UHMWPE Click here to View figure |
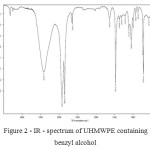 |
Figure 2: IR – spectrum of UHMWPE containing benzyl alcohol Click here to View figure |
A broad absorption band in the range 3200-3600 cm -1 confirms the presence of benzyl alcohol [6].
Thus, by IR – spectroscopy monitored process to obtain samples of UHMWPE containing nanoparticles of titanium oxides, zirconium and hafnium as well as by IR – spectroscopy were tested the samples to monitor the completeness of washing the organic products formed in the reaction of chlorides of titanium zirconium and hafnium with benzyl alcohol in the polymeric matrix UHMWPE.
In a study of UHMWPE samples containing the oxides of titanium, zirconium and hafnium XRF method was used to determine the presence of these metals in the composition of the samples. Thus, tools proved the presence of titanium, zirconium and hafnium as part of the experimental samples. X-ray spectrum analysis are shown in Figures 3 – 5.
 |
Figure 3: XRF spectrum of UHMWPE containing zirconium dioxide Click here to View figure |
 |
Figure 4: The XRF spectrum of UHMWPE containing hafnium oxide Click here to View figure |
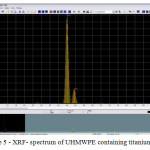 |
Figure 5: XRF- spectrum of UHMWPE containing titanium oxide Click here to View figure |
The study of structure-based materials UHMWPE containing oxides of titanium, zirconium and hafnium was examined by SEM surface of test specimens, as well as starting and intermediate products produced during the synthesis of the experimental samples. Figures 6 – 8 are shown pictures of UHMWPE samples containing oxide nanoparticles, as well as the source of UHMWPE obtained by SEM.
Image analysis allows us to conclude that on the surface of the polymer matrix contains agglomerates of particles as small as 2 microns, but in the lower layers clearly observed oxide nanoparticles with dimensions of the order of 100 nm or less, which is consistent with the position of preventing agglomeration of the particles in the polymer matrix.
 |
Figure 6: SEM image of the original sample of UHMWPE Click here to View figure |
 |
Figure 7: SEM image of a sample of UHMWPE containing nanoparticles of titanium dioxide Click here to View figure |
 |
Figure 8: Image SEM UHMWPE sample containing the nanoparticles of zirconium oxide Click here to View figure |
SEM was used to study the surface of the experimental samples of UHMWPE containing oxides of titanium, zirconium and hafnium. It was shown that the surface layers of the polymeric matrix along with the oxide nanoparticles are also present and larger agglomerates. The agglomerates of particles are measured in the range of 2-6 microns, the dimensions of individual filler particles do not exceed 50-100 nm (Fig. 9 – 11). This confirms the theoretical position that the preparation of oxide nanoparticles directly into the polymer matrix sintering process difficult [7].
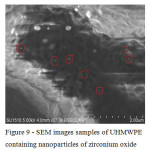 |
Figure 9: SEM images samples of UHMWPE containing nanoparticles of zirconium oxide Click here to View figure |
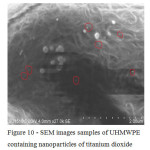 |
Figure 10: SEM images samples of UHMWPE containing nanoparticles of titanium dioxide Click here to View figure |
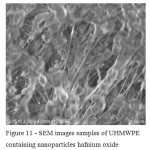 |
Figure 11: SEM images samples of UHMWPE containing nanoparticles hafnium oxide Click here to View figure |
Conclusion
During structural studies of composite materials containing nanoparticles of titanium, zirconium and hafnium oxides (TiO2, ZrO2 НfO2) by DSC, DRS, IR, XRF and SEM were examined properties of the obtained experimental samples and a number of intermediates. DSC were found new phase transitions in materials containing oxide nanoparticles show an increase in the onset temperature of melting. The method of the DRS determined the dielectric loss tangent in the material, found a marked increase in the dielectric loss of the samples containing metal oxides, the observed correlation between the properties of the materials – so increasing the dielectric loss as much as possible in samples containing hafnium oxide, they are also inherent in the maximum temperature rise of the beginning melting. By IR – spectroscopy confirmed the presence of benzyl alcohol molecules in the intermediates used to prepare test specimens on the basis of UHMWPE materials containing oxides of titanium, zirconium and hafnium. The same method was used to monitor the IR presence of organic impurities in the final samples.
With the use of X-ray analysis revealed the presence in the test specimens of titanium, zirconium and hafnium, so as to determine the chemical composition was obtained in the polymer matrix of the nanoparticles.
Acknowledgements
Applied researches are carried out with financial support of the state by the Russia Ministry of Education and Science under Grant Agreement № 14.576.21.0004 of June 17, 2014. (Unique identifier for Applied Scientific Researches (project) RFMEFI57614X0004).
References
- Andreev I., Veselovskaya E.V., Nalivaiko E.V., UHMW polyethylene high density. Chemistry, Leningrad (1982)
- Pakhomov P.M., Khizhnyak S.D., Golikova A., Galitsin В.P. Solid State Physics, 2005, 47(6), 994-999
- Niederberger M., Garnweitner G., Krumeich F., Nesper R. Chem. Mater., 2004, 16(7), 1202-1208
- Sutyagin V.M., Lyapkov A.A., Physical and chemical research methods of polymers. Tomsk Polytechnic University Publishing House, Tomsk (2008)
- Panin S.V., Panin V.E., Kornienko L.A., Puvadin T., Piriyaon S., Shilko S.V., News of Higher Schools. Series: Chemistry and Chemical Technology, 2011, 54 (7), 102-106
- Ginsburg O.F., Zavgorodniy V.S., Zubritskiy L.M., Pavlova L.A., Ral K. B., Sevbo D.P., Stadnichuk M.D. Workshop on organic chemistry synthesis and identification of organic compounds, Higher School, Moscow (1989)
- Pomogailo A.D. Colloid Journal, 2005, 67(6), 726-747

This work is licensed under a Creative Commons Attribution 4.0 International License.









