Solvolysis of titanium tetrachloride in non-aqueous media as a method for producing titanium dioxide particles of different morphology.
A. M Nemeryuk and M. M Lylina
Federal State Unitary Enterprise ”State Research Institute of Chemical Reagents and Pure Chemical Substances”(FSUE “IREA”), 107076, Russia, Moscow, Bogorodskiy val, 3
Corresponding Author Email: amnamn@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/310473
Article Received on :
Article Accepted on :
Article Published : 09 Dec 2015
The processes of solvolysis of titanium tetrachloride in nonaqueous media were studied. The influence of the conditions of solvolysis on the size and morphology of the particles of titanium dioxide produced have been described.
KEYWORDS:Тitanium dioxide; silica; titanium tetrachloride; non-aqueous media; triethylamine
Download this article as:| Copy the following to cite this article: Nemeryuk A. M. Lylina M. M. Solvolysis of titanium tetrachloride in non-aqueous media as a method for producing titanium dioxide particles of different morphology. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Nemeryuk A. M. Lylina M. M. Solvolysis of titanium tetrachloride in non-aqueous media as a method for producing titanium dioxide particles of different morphology. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13161 |
Introduction
Development of the scientific basis of catalyst preparation contributes to the creation of the original high-performance catalysts and methods for their synthesis. This work leads to a deeper understanding of the influence of the preparation method on the real structure and surface properties of the catalyst allows the connection between the structure and catalytic properties [1-3].
Influence of the reaction medium on the structure of the catalyst was studied in works Boreskov [1]. It is shown that the actual structure of the catalyst can be significantly altered by the action of the reaction medium. Stationary state of the catalyst surface of the same phase composition in many cases does not depend on the starting characteristics of the structure as dislocations and other disturbances present in the structure, as the high-energy state can disappear when the catalyst is contacted with the reaction medium. In the future, these provisions have been developed and refined in the work carried out under the leadership of Sadykov [3]. It was shown that in oxide systems with a low strength of the metal-oxygen (oxides of manganese, copper, cobalt, cobaltites and manganates), regardless of the initial structure of the catalyst under the influence of the reaction medium and elevated temperatures of structure rebuilt into one appropriate to the stationary state, determined by the composition of the reaction medium. For oxides with a sufficiently high bond strength of the lattice oxygen (oxides of titanium, nickel, chromium) defective structure is maintained at an elevated temperature and prolonged contact with the reaction medium. For such systems, characterized by only limited changes in the structure under the influence of the reaction medium. In this case, the active sites are localized on the surface of the ground where the lack of coordination atoms prevent the formation of strongly bound oxygen.
Analysis of the literature and our own data shows that the study of the real structure of oxide catalysts is important not only to establish the nature of the active component of the catalysts and catalytic reaction mechanism, but also for the development of the original catalyst compositions and methods for their synthesis. This makes it possible to create new materials with high activity and selectivity in catalytic processes, since communications can be identified unambiguously: synthesis catalyst structure, catalytic properties. In this regard, the study of the genesis of the real structure of oxide catalysts, depending on the method of cooking is one of the important tasks.
Also established unambiguously link the reactivity of oxides and other compounds with their chemical structure. By varying the degree of perfection of the crystal lattice of the starting material, and you can change the speed of its response to the solid-phase reactions. A characteristic feature of the flow of solid state reactions is their localization in certain places reacting solid. The predominance of one or another form of localization of the reaction and the nature of its course, in most cases related to the features of the crystal structure of the starting materials and reaction conditions. Active sites are usually associated with the formation of structural defects in crystals and concentrated in certain crystallographic directions. Therefore, the presence of various kinds of disorders of a solid, usually accounts for its greater reactivity.
Among the materials exhibiting catalytic activity on oxidation reactions allocated titanium dioxide and oxide system based on it, containing silicon oxides of aluminum, iron, vanadium, molybdenum, tungsten, antimony and other elements, methods for the preparation of which, as well as bond the composition and morphological characteristics of with a catalytic activity intensively investigated [4-12].
A considerable number of techniques for preparing such systems based on the hydrolytic processes in aqueous media or seine occurring by reacting titanium compounds such as alkoxides or halides, with water or other compounds – oxygen donors [13].
One method for producing titanium dioxide in nonaqueous media is the reaction of titanium halides, as a rule – titanium tetrachloride or titanium alkoxides with aromatic fatty alcohols such as benzyl alcohol in organic solvent under anhydrous conditions. This method was first described in the works related to the preparation of nanoparticles of titanium dioxide and other elements [14-18].
We have studied the formation processes of titanium dioxide particles in non-aqueous media by reacting titanium tetrachloride with benzyl alcohol at different process conditions.
So, it was used to study the interaction between benzyl alcohol with titanium tetrachloride in the presence of an acceptor of hydrogen chloride – triethylamine under heterogeneous conditions in a medium of n-decane, also investigated the process under similar conditions, but without using triethylamine addition was investigated the interaction of titanium tetrachloride and benzyl alcohol in a homogeneous process conditions in the environment of o-xylene. It was also examined the possibility of introducing the silica into the titanium dioxide particles obtained by introducing into the reaction mixture of tetramethoxysilane during the alcoholysis reaction. The samples obtained oxides were investigated by X-ray diffraction, scanning electron microscope and the particle size distribution. It was found that the conditions of the process of obtaining oxide particles have a significant impact on the morphological characteristics of the resulting product.
Material and Methods
Preparation of titanium dioxide containing silica in a medium of n-decane in the presence of triethylamine.
To a solution of 0.2 mol (38g, 22.3 ml) of titanium tetrachloride in 500 ml of n-decane with vigorous stirring was added dropwise a mixture of 110 mL of triethylamine (1.1 mol) and 75 ml (0.4 mol) benzyl alcohol temperatures not above 40 ° C. Observed the formation of a white precipitate. To the reaction mass been added 5 ml of tetramethoxysilane. The reaction mixture was heated at reflux for 8 hours. The precipitate was filtered and washed successively with isopropanol and chloroform. Obtained 21.4 g of product. Approximately 0.9 grams of product obtained was calcined at 850 ° C for 10 minutes in an air atmosphere for the purpose of thermooxidative destruction of organic impurities.
Preparation of titanium dioxide in a medium of n-decane – benzene 1: 5
With vigorous stirring, to a solution of 0.02 mol of titanium tetrachloride in 100 ml of n-decane was dissolved is added 0.04 mol of benzyl alcohol in 20 ml of benzene at a temperature not higher than 40°C. There was a release of hydrogen chloride and the formation of dark colored yellow emulsion. The reaction mixture was heated at reflux for 4 hours, the conversion was observed in the emulsion slurry and loss of dark yellow. Then, benzene was distilled off from the reaction mixture and continued refluxing at the boiling temperature for 8 hours. The precipitate was filtered and washed with chloroform. Obtained 1.8 g of product.
Preparation of titanium dioxide in the medium o- xylene
To a solution of 0.02 mol of titanium tetrachloride in 100 ml of o-xylene under vigorous stirring and at a temperature not higher than 40°C is added the solution was 0.04 mol of benzyl alcohol in 20 ml of o-xylene. The reaction mixture was heated at reflux temperature for 8 hours. When heated, hydrogen chloride evolution was observed, and the formation of sludge. Initially lilac color of the reaction mixture changed to light yellow. The precipitate was filtered off and repeatedly washed with chloroform. Obtained 2 g of product.
Results and Discussion
The resulting samples were examined by SEM. Detected difference in the particle morphology, the particles obtained in the course of the experiment titanium dioxide in a medium of n-decane in the presence of triethylamine had porous structure (figure 1 and 2).
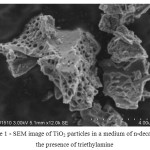 |
Figure 1: SEM image of TiO2 particles in a medium of n-decane in the presence of triethylamine |
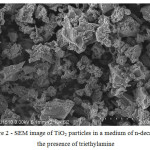 |
Figure 2: SEM image of TiO2 particles in a medium of n-decane in the presence of triethylamine |
The titanium dioxide particles obtained without using triethylamine, in a medium of n-decane-benzene did not have, according to the SEM of the microporous structure, and the particles were agglomerates of fine flattened shape (figure 3).
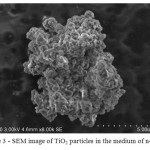 |
Figure 3: SEM image of TiO2 particles in the medium of n-decane Click here to View figure |
The study SEM titanium dioxide particles obtained in the medium of xylene, found that they are rounded and much smaller than those obtained in the other, above-described conditions.
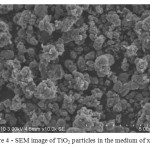 |
Figure 4: SEM image of TiO2 particles in the medium of xylene Click here to View figure |
XRD data were obtained for all the samples studied.
It should be noted that there existents crystalline anatase phase, as well as the presence in the sample of silica (figure 5).
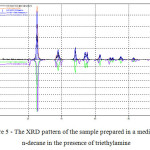 |
Figure 5: The XRD pattern of the sample prepared in a medium of n-decane in the presence of triethylamine |
The size of the particles was determined using the instrument Mastersizer.
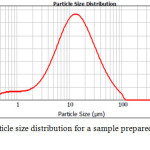 |
Figure 6: The particle size distribution for a sample prepared in a medium of n-decane Click here to View figure |
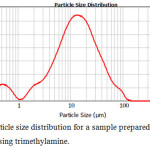 |
Figure 7: The particle size distribution for a sample prepared in a medium of n-decane-benzene using trimethylamine. Click here to View figure |
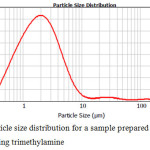 |
Figure 8: Тhe particle size distribution for a sample prepared in a medium of o-xylene without using trimethylamine Click here to View figure |
Conclusion
The study has revealed the dependence of the size and morphological characteristics of the particles to the conditions of the alcoholysis of titanium tetrachloride with benzyl alcohol in nonaqueous media. It is noteworthy that the use of triethylamine as the acceptor of hydrogen chloride leads to obtaining a microporous structure, whereas during the process in similar conditions but without the use of an organic amine as a hydrogen chloride acceptor are formed of titanium dioxide particles of similar size and shape, but devoid of pores. Conducting the interaction of titanium tetrachloride with benzyl alcohol under homogeneous conditions in a medium of o-xylene leads to the formation of titanium oxide particles and substantially round shape (almost an order of magnitude) smaller. Thus, the monitoring method allows precise control of the size and morphological characteristics of titanium dioxide particles.
Acknowledgements
Applied researches are carried out with financial support of the state by the Russia Ministry of Education and Science under Grant Agreement No.14.624.21.0006 of September 8, 2014. (Unique identifier for Applied Scientific Researches (project) RFMEFI62414X0006).
References
- Boreskov G.K., Heterogeneous catalysis; Nauka. Moscow (1986)
- Krylov O.V. Kinetics and Catalysis 2000, 40(5), 752
- Sadykov V.A. Dis. Doctor. chemical. Sciences, Novosibirsk, Institute of Catalysis. GK Boreskov SB RAS, 1998, 410
- Choi H.-J., Kim J.-S., Kang M. Bull. Korean Chem. Soc. 2007, 28, 581
- Li H., Bian Z., Zhu J., Huo Y., Li H., Lu Y. J. Am. Chem. Soc. 2007, 129, 4538
- Valentin C. D., Pacchioni, G., Selloni A. Chem. Mater. 2005, 17, 6656
- Usseglio S., Damin A., Scarano D., Bordiga S., Zecchina A., Lamberti C. J.Am. Chem. Soc. 2007, 129, 2822
- Morandeira A., Lopez-Duarte I., Martinez-Diaz M. V., O’Regan B., Shuttle C., Haji-Zainulabidin N. A., Torres T., Palomares E., Durrant J. R. J. Am. Chem. Soc. 2007, 129, 9250
- Tang J., Quan H., Ye J. Chem. Mater. 2007, 19, 116
- Zan L., Liu Z., Zhong J., Peng Z. J. Mater. Sci. 2004, 39, 3261
- Sójka-Ledakowicz J., Lewartowska J., Kudzin M., Leonowicz M., Jesionowski T., Siwińska-Stefańska K., Krysztafkiewicz A. J. Mater. Sci. 2009, 44, 3852
- Yoon M., Seo M., Jeong C., Jang J. H., Jeon S. K. Chem. Mater. 2005, 17, 6069
- Kongsuebchart W., Praserthdam P., Panpranot J., Sirisuk A., Supphasrirongjaroen P., Satayaprasert C. J. of Crystal Growth 2006, 297, 234
- Rockenberger J., Scher E. C., Alivisatos A. P. J. Am. Chem. Soc. 1999, 121, 11595-11597
- Shim M., Guyot-Sionnest P. J. Am. Chem. Soc. 2001, 123, 11651-11654
- Joo J., Yu T., Kim Young W., Park H. M., Wu F., Zhang J. Z., Hyeon T. J. Am. Chem. Soc. 2003, 125, 6553-6557
- Jun Y.-W., Casula M. F., Sim J.-H., Kim S. Y., Cheon J., Alivisatos A. P. J. Am. Chem. Soc. 2003, 125, 15981-15985
- Niederberger M., Garnweitner G., Krumeich F., Nesper R., Cölfen H., Antonietti M. Chem. Mater. 2004, 16(7), 1202-1208

This work is licensed under a Creative Commons Attribution 4.0 International License.









