Rapid Kinetics and Relative Reactivity of Some Five Membered Aromatic Heterocycles using Hydrodynamic Voltammetry
S. B. Walke, S. L. Bonde*,R. P. Bhadane, V. T. Dangat and B. Jadhav
Department of Physical Chemistry, Nowrosjee Wadia College, affiliated to Savitribai Phule Pune University, India.
Corresponding Author Email: sbonde52@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310449
Article Received on :
Article Accepted on :
Article Published : 02 Dec 2015
Kinetics of the bromination of imidazole, pyrazole and thiazole by molecular bromine and N-bromosuccinimide has been studied in aqueous medium. Since the reactions are rapid special technique namely, hydrodyanamic voltammetry has been employed to follow the course of the reactions. These reactions follow second order kinetics. The comparative kinetic data determines the reactivity order for these heterocycles towards the bromination using two different brominating reagents. The study justifies the stereochemical principles ascertaining the relative reactivity of these heterocycles quantitatively using kinetics as an investigational tool.
KEYWORDS:Kinetics; rapid bromination; rotating platinum electrode; steriochemical principles
Download this article as:| Copy the following to cite this article: Walke S. B, Bonde S. L, Bhadane R. P, Dangat V. T, Jadhav B. Rapid Kinetics and Relative Reactivity of Some Five Membered Aromatic Heterocycles using Hydrodynamic Voltammetry. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Walke S. B, Bonde S. L, Bhadane R. P, Dangat V. T, Jadhav B. Rapid Kinetics and Relative Reactivity of Some Five Membered Aromatic Heterocycles using Hydrodynamic Voltammetry. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12817 |
Introduction
Imidazole, pyrazole and thiazole are planar five membered heterocyclic compounds. Many heterocyclic compounds have wide range of applications in chemical, pharmaceutical, biological and material sciences1-4. These compounds are highly reactive in electophilic substitution reactions. The halogenation of these heterocycles is of great interest. The bromination of aromatic and heteroaromatic compounds is observed to be important in synthetic organic chemistry5. The bromo derivatives of these heterocycles are used as synthetic intermediates in transition metal catalyzed cross coupling reactions and in pharmacologically active compounds6, 7. It therefore seems likely that the study of kinetics and mechanism of such reactions would be useful in these applications. The present work deals with the quantitative assessment of the relative reactivities of three heterocycles with respect to two brominating agents, namely molecular bromine and N-bromosuccinimide (NBS). Among halogenations bromination is observed to be the fastest8.The reactions are observed to be very rapid to study by conventional methods. The rapidity of such reactions necessitates the use of special techniques such as stopped flow method, temperature jump method and potentiometric methods9-11.We have herein employed simple yet useful technique i.e. hydrodynamic voltammetry12. The rotating platinum electrode (RPE) is used to monitor the course of rapid reaction. Kinetics of some rapid halogenations has been studied by using RPE13-15. Previously we have studied the kinetics of base catalyzed iodination of imidazole and 2-methylimidazole by molecular iodine and chlorination of imidazole and its regioisomers by molecular chlorinein aqueous medium by the RPE technique16, 17. The present study comprises the bromination of imidazole (I), pyrazole (II) and thiazole(III) by two different reagents, molecular bromine and N-bromosuccinimide (NBS) in aqueous medium using RPE. These electrophilic substitution reactions follow second order kinetics. The extent of reaction is monitored by following the decrease in diffusion current at the rotating platinum cathode versus saturated calomel electrode (SCE). The diffusion current measured is a function of decaying concentration of Br2 or NBS at various time intervals. Since the reactions follow second order kinetics their half lives can be extended by diluting the solutions thus the convenient kinetic measurements of such rapid reactions are feasible. The dilute solutions are used due to rapidity of reactions and the green chemistry principles are inherent in this study18. The use of large concentration of the supporting electrolyte, KNO3 ensures linear proportionality of the diffusion limited current at the RPE due to unreacted Br2 or NBS concentration in the cell. The kinetics of bromination of imidazole has also been carried out at various temperatures using NBS in aqueous medium to determine the kinetic parameters such as the energy of activation, frequency factor, enthalpy of activation and entropy of activation 19.
The reactions under study are shown as follow:
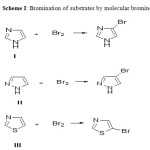 |
Scheme 1 Click here to View scheme |
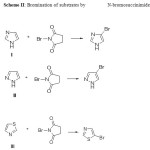 |
Scheme 2 |
Experimental
Materials
Imidazole, pyrazole, thiazole, and N-bromosuccinimide of analytical grade chemicals were used in present study to prepare the stock solutions of required concentrations. All chemicals were purchased from Sigma–Aldrich. Molecular bromine is obtained by dissolving the bromine pellets in double distilled water. The exact concentration of bromine solution was determined by iodometric titration method using starch as an indicator and diluted to required concentrations. Solution of bromine and NBS each contains hundred folds potassium nitrate as supporting electrolyte. Potassium iodide, sodium thiosulphate and starch of analytical grade were used in standardization of Br2 solution.
Calibration of diffusion current
The diffusion current due to bromine was calibrated in terms of deflection of light spot with the help of galvanometer and scale arrangement. The galvanometer deflection was measured on scale in cm unit which was converted in unit of nA. The diffusion current values were recorded at various concentrations of Br2 and NBS in their required range the data are given in table 1. The linear plot was obtained for the diffusion current Vs the concentration of Br2 or NBS.
Kinetic Measurements
Equimolar solution of imidazole (I) and bromine containing 100 folds potassium nitrate were poured simultaneously in a beaker assembled with RPE and SCE after attaining the thermostat temperature. At the moment of mixing a stopwatch was started. The extent of reaction was measured by recording the diffusion current at various time intervals. The concentration of unreacted bromine at this time intervals was determined from the calibration curve observations are summarized in table 2. The above procedure of calibration and kinetic measurement was repeated twice for checking the reproducibility of the galvanometer measurements, and these were found to be within the limits ±0.2 cm. The reciprocal of [Br2] versus time was plotted (figure 1). A straight line was obtained since the reaction is of second order; the slope of this plot gives specific reaction rate k2. The kinetics measurements were repeated for bromination of pyrazole (II) and thiazole (III) by molecular bromine and also for bromination of substrates I, II and III by N-bromosuccinimide data is summarized in figure 1and 2.
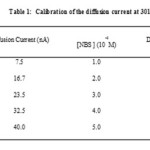 |
Table 1 |
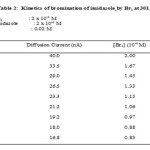 |
Table 2 |
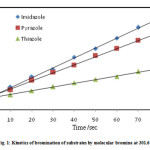 |
Figure 1 |
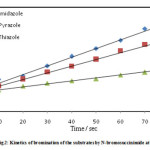 |
Figure 2 |
Results and Discussion
The second order kinetics was observed for the bromination of imidazole, pyrazole and thiazole using molecular bromine and N-bromosuccinimide in aqueous medium since the linear plot is obtained for [Br2]-1 or [NBS]-1Vs time. The slope of the plot gives the specific reaction rate. The results are summarized in Table 3. The reaction follows electrophilic substitution pathway and is observed to be rapid. The special technique hydrodynamic voltammetry is employed to monitor the progress of the reaction.
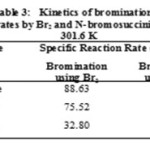 |
Table 3 |
The kinetic measurements have an error of less than + 2.0% in view of the reproducibility of the diffusion current. The order of the magnitude of the specific reaction rates as kimidazole >kpyrazole >kthiazole concludes their relative reactivity towards the bromination using molecular bromine or NBS in aqueous medium. The bromination of these substrates are observed to be faster when Br2 is used as brominating reagent compared to those when NBS is used. Principles of stereochemistry have been quantitatively justified. Imidazole and thiazole both are 1, 3 azole, but there is difference between the heteroatom in rings. The sulphur substitution for NH dramatically reduces basicity of thiazole. Thiazole rings are characterized by larger Π electron delocalization and have therefore greater aromaticity than that of imidazole and pyrazole. Also in thiazole the nitrogen atom deactivates the ring towards electrophilic substitution like in pyridine. Delocalization and stabilization in thiazole being the greatest among the three substrates, thus the reactivity towords the electrophilic substitution of thiazole is observed to be less. Thus the rate constant for bromination of thiazole is observed to be less than that of the imidazole and pyrazole. Comparison between 1,3 azoles and 1,2 azoles shows that the 1,2 azoles (Pyrazole) are less reactive than the 1,3 azoles (Imidazole). Since the nitrogen atoms at 1, 2 position in pyrazole are adjacent and they highly attracts ∏ electron clouds towards them and observed to be more aromatic than 1,3 azole (imidazole). The imidazole is much more basic than pyrazole because of this base weakening interaction of adjacent N in pyrazole.20 Hence pyrazole are less reactive and rate constant also observed to be less as compared to imidazole.
The values of specific reaction rates for the bromination of these substrates using NBS are found to be lower than those of bromination using molecular bromine at the same temperature. The kinetic data quantitatively justify the relative nucleophilicity of substrates towards the two different brominating reagents in the electrophilic substitution reactions. In bromination using molecular bromine, the Br2 is the sole brominating reagent in the reaction in view of the equilibrium Br2 + H2O ↔ HOBr + HBr which in aqueous solution is predominantly shifted to the left. The hydrolysis constant is very lowand since the hypohalous acid is known to be very weak electrophile.21 Molecular bromine in the presence of water induces a dipole moment at one end of the bromine molecule. The induced positive charge of bromine is more electrophilic as compared to the positive charge on the bromine of NBS. Hence the bromination is observed to be faster in the case of molecular bromine is used. Comparatively the NBS is a bulkier group than Br2. When the electrophile is relatively less bulky the steric hindrance does not affect the reaction rate markedly. Steric compulsion stems from the bulkiness of NBS that reacts with the substrates. This is less marked when Br2 is used instead of NBS. Thus the observed order of magnitude of specific reaction rates determined for the bromination of substrates under study justifies the predicted relative reactivity of the two brominating reagents in aqueous solution in electrophilic aromatic substitution reactions. The mono-chloro products are formed in each case at the positions that are stereo chemically less hindered.
The study of kinetics of bromination of imidazole by N-bromosuccinimide has also been subjected to various temperature ranges from 287.1K to 306.2K. The specific reaction rates are given in table 4. Various activation parameters such as energy of activation, enthalpy of activation and entropy change are evaluated results of which are summarized in table5.
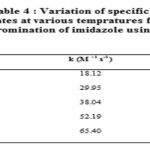 |
Table 4 |
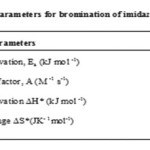 |
Table 5 |
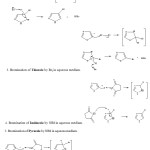
The mechanism proposed for the bromination of the substrates by Br2 and N-bromosuccinimide follows the electrophilic substitution pathway. The polarization of reagent takes place in polar solvent to generate the electrophile Brd+. The pi electron clouds of the respective double bond in the substrates attack the Brd+. The substrate molecule losses its aromaticity and becomes unstable. The -ve end of the reagent
molecule abstracts the proton in the fast step to stabilize the product in order to regain the aromaticity. The mono bromo derivatives are formed in each case confirmed stochiometrically. Previous workers have reported that the electrophilic substitution in imidazole takes place at the 4(5)position and the C-2 substitution is highly unfavored.22 In pyrazole electophilic substitution takes place at position 4.23 While in thiazole the position 5 is more prone to substitution than the 4-position while 2-position is far less susceptible to electophilic attack.24
Scheme III: The probable mechanism for the bromination of the substrates under study:
 |
Scheme 3 |
Conclusion
The kinetics of bromination of the five membered aromatic heterocycles studied follow second order kinetics and are too rapid to study by conventional methods. The special technique, hydrodynamic voltammetry is employed to follow the progress of the reactions. The observed behavior of rate constant is in accordance with stereochemistry principles. The highest rate is observed for the bromination of imdazole compared to the pyrazole and thiazole. Also the bromination is observed to be faster for the substrates when molecular bromine is used as brominating reagent compared to that of N-bromosuccinimide is used. The verification of the quantitative assessment of the relative reactivity of heterocycles is provided by this study of kinetics of bromination in aqueous solutions.
References
- Kumar, A. K.; Jayaroopa, P. International Journal of PharmTech Research,2013, 5(4),1473-1486
- Kumari, S.; Sharma, P. K.; Kumar, N. Pelagia Research Library, 2010, 1(3), 36-47
- Bhatnagar, A.; Sharma, P. K.; Kumar, N. International Journal of PharmTech Research, 2011, 3(1), 268-282
- Sheng, F.; Wang, Y.; JingSong, L. V.; Damu, G.; ChengHe, Z. Scientia Sinica Chimica, 2012, 42(8), 1105- 1131
- Larock, R. C.; Comprehensive organic Transformations: A Guide to Functional Group Preparations 1 st Ed Wiley VCH New York 1997
- Li, G.; Kakarla, R.; Gerritz, S. W. Tetrahedron Lett. 2007, 48, 4595-5499
- Salaheldin, A. M.; Oliveira, Campos F.; Rodrigues, L. M. Tetrahedron Lett. 2007,48, 8819- 8822
- Dangat, V. T.; Bonde, S. L.; Rohokale, G. Y. Indian Journal of Chemistry 1984 23A ,237-238
- Patil, D. B.; Thakur, G. J.; Shende P.M. Oriental Journal of Chemistry, 2011, 27(1) 197-202
- Hart, E. J.; Boag, J. W. J. Amer. Chem. Soc. 1963, 84, 4090
- Atkinson, J. R.; Bell, R. P. J.Chem Soc. 1963, 3260-3269
- Kolthoff, I. M.; Lingane, J. J. Polarography 2nd Edition Interscience Publishers New York , 1952 1, 421
- Borkar, V. T.; Bonde, S. L.; Dangat, V. T. International Journal of Chemical Kinetics 2013,45(11), 693–702
- Bell, R. P.; Spencer, T. Proc. Royal Soc 1959 A 251
- Dangat, V. T.; Bonde, S. L.; Borkar, V. T.; Maske, P. D Research Journal of Chemical Sciences 2012, 2(7), 75-78
- Walke, S. B.; Bonde, S. L.; Dangat, V. T.; Bhadane, R. P. International Journal of Science and Research 2015, 4(6), 2890-2894
- Bonde, S. L,.; Dangat, V. T.; Bhadane, R. P.; Joshi, V. S. International Journal of Chemical Kinetics 2013, 45(6) , 355- 362
- Pandey, B.; Fulekar, M. H. Research Journal of Chemical Sciences 2011, 1 (1), 111-117
- Maron, S.; Prutton, C. Principle of Physical Chemistry 4 th edn. p. 573, 584-585
- Katritzky, A. R.; Pozharski, A. F. Handbook of Heterocyclic Chemistry 2nd Edn. Pergamon / Elsevier 2000
- Berliner, E. J, Chem .Edu. 1966, 43, 124
- Bhatnagar, A.; Sharma, P. K.; Kumar, N. International Journal of PharmTech Research 2011, 3(1), 268-282
- Schofield Kenneth; Grimmett, M. R.; Richard Brian Thornton Keene Heteroaromatic Nitrogen Compounds: The Azoles Published by Cambridge University Press, 1976 p 45- 48
- Begtrup, M.; Hansen, L. B. L; Acta Chem. Scand. 1992, 46, 372-383

This work is licensed under a Creative Commons Attribution 4.0 International License.









