Preparation and Characterization of Activated Carbon from Coconut Shell - Doped Tio2 in Water Solution
Maulidiyah, Dwiprayogo Wibowo, Hikmawati, Richard Salamba and Muhammad Nurdin*
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Halu Oleo, Kendari 93232 – Southeast Sulawesi, Indonesia
Corresponding Author Email: mnurdin06@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310462
Article Received on :
Article Accepted on :
Article Published : 10 Nov 2015
The objective of the study was to prepare the activated carbon material doped TiO2-P25 (P25)in order to determine the interaction occured in the water medium. The method was to prepare the activated carbon from coconut shell which had been cleaned, pyrolyzed, sievedthen followed by physical activation using a thermal process. Preparation of P25 was to form structures of anatase crystals in the furnace at temperature of 500°C for 3 hours. Both materials were mixed using distilled water until sol-gel was formed. Results of characterization using SEM showed that there is interaction between the activated carbon and P25inserted in the pores of the carbon, while SEM-EDX showed the composition of carbon, titanium and oxide are 46.9%; 27.5% and 25.6%, respectively. Data from XRD showed the formation of peaks from P25 anatase crystals and the carbon. It was supported by data of Flourier Transform Infra Red (FTIR) which showed the bonds of –OH; C-H; C=C; COand the O-Ti-O.
KEYWORDS:Active Carbon; TiO2; Characterization; Water; Coconut Shell; Doped
Download this article as:| Copy the following to cite this article: Maulidiyah, Wibowo D, Hikmawati, Salamba R, Nurdin M. Preparation and Characterization of Activated Carbon from Coconut Shell - Doped Tio2 in Water Solution. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Maulidiyah, Wibowo D, Hikmawati, Salamba R, Nurdin M. Preparation and Characterization of Activated Carbon from Coconut Shell - Doped Tio2 in Water Solution. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12571 |
Introduction
Titanium dioxide (TiO2) photocatalytic process is a promising process to overcome water pollution due to the use of oxidation-reduction reactions triggered by excitation of electrons under photon radiation (light) with greater energy to activate the band gap of TiO2. Photogenerationprocess of electron pair (e–) and hole (h+) can be recombined, so if there is no recombination process, the pair of electrons can migrate to the surface of TiO2 to participate in the process of reduction-oxidation reactions (redox), each of which allows the formation of superoxide anion radical (•O2–) and hydroxyl radical (•OH)1-3.
TiO2 is selected as one of semiconductor materials due to it is inert to chemicals, non-toxic and unexpensive in its utilization4. TiO2 has been used in various application fields such as for overcoming environmental pollution, self-clean, paint industry, photoelectric sensors, anti-fungal material, photovoltaic cells as well as filtering materials5. However, the disadvantages of TiO2 compound are it is active only on irradiation of UV light with the band gap value of 3.2 eV, so various researches have been developed to change the TiO2 band gap by inserting non-metal element, while trapping electrons by inserting metal elements6,7. The process of doping on the TiO2 semiconductor has an objective to change the properties of TiO2 in order to make it works in visible light (low-energy) to be applied to the use of sunlight.
Various doping methods development of TiO2 compounds lead to the utilization of very expensive, high toxicity and the difficulty prepared materials8. So in this study, it led to the utilization of organic waste such as coconut shell which can encourage the development of new researches combined with P25 compounds using aqueous solvent, which have been developed based on TTIP material as a precursor of TiO29. Coconut shell material can be made into charcoal that has excellent resistance since it has been widely used as harmful metal adsorbent media such as Mn, Zn, Cd and Pb10, whereas the TiO2 compounds can be used as water purification from bacteria because of its photo active property resulting in the molecule breakdown of organic compounds in the water which makes it safe to consume11-14.
Therefore, in this study preparation and characterization of activated carbon from coconut shell doped by P25 was performed to identify the utilization of conventional solvent, i.e distilled water to determine the interaction of both materials using distilled water which is one of the promising method developments in improving the doped TiO2 semiconductor.
Experimental
Preparation of Carbon from Coconut Shell
Coconut shell, taken from the copra processed waste in the region of Southeast Sulawesi, was cleaned up of its fiber on shell using knife. Subsequently the shell was made into small pieces in size of 3-5 cm, sun-dried and aerated in order to reduce the water content of samples so it would produce good carbon.
CarbonationandActivationof Coconut Shell
Carbonation stage of coconut shell was done by conducting pyrolysis process at a temperature of ± 700ºC for 1 hour. Result of coconut shell charcoal carbonation was grinded dan sieved using a 170 mesh sieve. Charcoal was then activated in termolitte furnace with a temperature of 500ºC and simultaneously nitrogen gas was purged to produce coconut shell activated carbon.
Synthesis of Carbon–doped TiO2
Synthesis of coconut shell activated carbon doped P25 was done by performing stoichiometricly ratio of 1: 1, where the P25 was calcined in a furnace at a temperature of 500ºC to form anatase crystal then mixed in a beaker with the addition of distilled water. Microscopic of activated carbon doped P25 was observed using SEM, SEM-EDX, XRD and FTIR.
Results and Discussion
Sample Preparation of Coconut Shell
The collecting of raw material, coconut shell, was taken from the copra processed waste in the region of Southeast Sulawesi. The collecting process was performed by selecting dried coconut shell and had good thickness to make shorter cleaning process, save time and to have good resistance to heat. Coconut shell was then cleaned thoroughly from coconut fiber attached to surface of shell. The final stage of raw material preparation was sun drying the coconut shell and left it overnight to produce dried shell to make it easy in the process of carbonation.
Carbonation and Activation of Coconut Shell
Carbonation was performed by pyrolysis process at a temperature of ± 700ºC for 1 hour and the results of the pyrolysis process produced quite hard charcoal. According Jahirul et al. pyrolysis at temperatures >500ºC produces endurance and excellent charcoal crystal structure15. Furthermore charcoal obtained was grinded to produce fine-grained charcoal, this technique can produce small particles, the smaller the particle, the greater surface area of particle16,17. After the grinding process, then the next stage was sieving which aimed to produce charcoal powder with the same particles (170 mesh). Technique of charcoal activation was done using physical methods performed by heating process within the termolitte furnace which simultaneously purged with nitrogen gas, expected to produce broader pore size so it can be used as binding medium between P25 compounds to be synthesized.
Synthesis of Coconut Shell Activated Carbon doped TiO2
Activated carbon and P25 powder was weighed of 2.5 grams and put into a 100 mL beaker with the addition of a little distilled water, visually of this process, it produced colloid as shown in Figure 1.
 |
Figure 1: Mixing ofactive carbon with TiO2 in water medium |
The process of activated carbon doped P25 was to determine the interaction between the activated carbon and P25 in water medium. This process was based on solid state reaction using easy conventional solvent. Moreover, the addition of TiO2 in carbon served as pillarization of the structure, extending the carbon surface so that it has excellent adsorption18 and improving the properties of TiO219. Huanget al. reported the bond formation between the carbons doped TiO2 tends to form carbon bond interstitially instead of substitutionly20. Application of materials doped TiO2 can be used in the adsorbent process in water treatment to eliminate bacteria contained in the water11. This is because TiO2 is a semiconductor which has photocatalysis property to solve various environmental problems among others are for water and air purification, destruction of microorganism such as bacteria and viruses, inactivation of cancer cell, degradation of dye and toxic chemical compounds and manufacture of hydrogen gas from water21. TiO2 semiconductor in photocatalytic oxidation process has an advantage in overcoming environmental pollution caused by dye7.
Characterization
Scanning Electron Microscope (SEM)
Sample preparation of activated carbon doped P25 showed that there had been coating. The distribution of activated carbon to P25 indicated that TiO2 filled the pores of activated carbon. Figure 2 shows the particle size of activated carbon and P25 resulted90 μm and 1 μm, respectively. So, it could be inserted between pores of activated carbon. It could be also assumed that the possibility of P25 can be pillarization and enlarge the pores of activated carbon so it enabled bond formation between C-O-Ti-O.
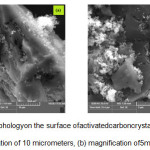 |
Figure 2: Morphologyon the surface of activated carboncrystaldoped P25; a). Magnification of 10 micrometers, (b) magnification of 5 micrometers |
Scanning Electron Microscopy – Energy Dispersive X-ray Spectroscopy (SEM – EDX)
SEM-EDX data showed the morphology of synthesized materials from activated carbon doped P25 (Figure 3) showed the composition of the presence of carbon compounds dopedP25. From the result of the spectrum, it showed the presence of Ti (Titanium) elements on energy bands, i.e 4.25 KeV and 0.20 KeV. From the sample, it still showed a wide range of metals which enable material to be dispersed in samples of P25 which was extracted from ilmenite natural materials22. This material showed three elements (C, Ti and O), the percentage of each element were 46.9%; 27.5% and 25.6%, respectively.
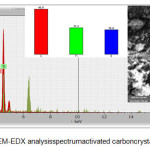 |
Figure 3: SEM–EDX analysis spectrum activated carboncrystaldoped P25 |
X-Ray Diffraction (XRD)
Preparation of activated carbon doped P25 was calcined at temperatures of 500°C aimed to form anatase crystals of TiO2 which is photoactive, as well as to open the pores of activated carbon. From crystal analysis using XRD formed from activated carbon doped P25 (Figure 4) the formation of carbon peaks on the field of 002 while TiO2 of anatase crystal shown in fields of 101, 004, 200, 220, 105, 204, 116, 215 and the persistence of the rutile crystal shown in fields of 110, 101, 210, 211, 002, 110 formed on the prepared sample. These indicated that the sample has been doped and interactions took place both on the surface of activated carbon and TiO2.
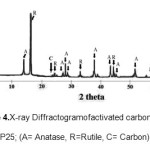 |
Figure 4: X–ray Diffractogram of activated carbondoped P25; (A= Anatase, R=Rutile, C= Carbon) Click here to View figure |
Flourier Transform Infra Red (FTIR)
Characterization using FTIR showed that based on the spectrum (Figure 5) it was indicated the presence of –OHgroup stretching at wavenumber of 3500 cm-1 (stretching vibration) with low signal. Similarly, at wavenumber of 2924 cm-1, it showed C–H bonds of the aromatic ring with moderate intensity. Then the wavenumbers of 1645 cm-1–1678 cm-1 showed the C=C bonds of an alkene compound type, while 1516 cm-1–1541 cm-1 showed the C=C bonds of the aromatic ring compound type, this issupported by the presence of C–O at wavenumber of 1112 cm-1. The presence of P25 at wave numbers of 516 cm-1–677 cm-1.
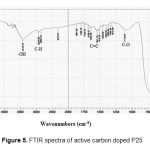 |
Figure 5: FTIR spectra of active carbon doped P25 Click here to View figure |
Conclusion
By using conventional solvent (distilled water), it resulted in the formation of colloidal activated carbon from coconut shell doped P25 and had been characterized using SEM, and the presence of interactions between activated carbon and P25 with a particle size are 90 μm and 1 μm, respectiely. The composition of carbon, titanium and oxide by SEM-EDX are 46.9%; 27.5% and 25.6%, respectiely. XRD data on the formation of P25 anatase crystals in the fields of 101, 004, 200, 220, 105, 204, 116, 215 and the carbon peak in the fields of 202. It was supported by FTIR data which was indicated –OH bond (3500 cm-1); C–H (2924 cm-1); C=C (1645 cm-1–1678 cm-1) and (1516 cm-1–1541 cm-1); C–O (1112 cm-1) and the O-Ti-O (516 cm-1–677 cm-1).
Acknowledgement
Our thanks to Ditlitabmas-Ministry of Research, Technology and Higher Education for financial support.
References
- Feng, T.; Feng, G.S.; Yan, L.; Pan, J.H. International Journal of Photoenergy. 2014,2014,1-14.
- Maulidiyah; Nurdin, M.; Widianingsih, E.; Azis, T.; Wibowo, D.ARPN Journal of Engineering and AppliedSciences. 2015, 10, 6250-6256.
- Jung, J.-Y.; Lee, D.; Le, Y.-S. Journal of Alloys and Compounds. 2015, 622, 651-656.
- Maulidiyah; Nurdin, M.; Erasmus; Wibowo, D; Natsir, M; Ritonga, H.; Watoni, A.H. International Journal of ChemTech Research. 2015,8, 416-423.
- Larumbe, S.; Monge, M.; Gomez-Polo, C. Applied Surface Science. 2015,327, 490-497.
- Nurdin M.International Journal of Pharma and Bio Sciences,2014, 5, 360-369.
- Maulidiyah; Nurdin, M.; Wibowo, D.; Sani, A. International Journal of Pharmacy and Pharmaceutical Sciences.2015,7, 141-146.
- Wu, L.; Li, F.; Xu, Y.; Zhang, J.W.; Zhang, D.; Li, G.; Li, H. Applied Catalysis B: Environmental. 2015, 164, 217-224.
- Rahman, M.M.; Choudhury, F.A.; Hossain, M.d.D.; Chowdhury, M.d.N.I.; Mohsin, S.; Hasan, M.d.M.; Uddin, M.d.F.; Sarker, N.C. Journal of Chemical Engineering. 2012, 27, 65-71.
- Paull, B.; Nesterenko, P.; Nurdin, M.; Haddad, P.R. Analytical Communications. 1998, 35, 17-20.
- Gupta, K.; Singh, R.P.; Pandey, A.; Pandey, A. Beilstein Journal of Nanotechnology. 2013, 4, 345-351.
- Zhao, X.; Zhang, J.; Qu, J. Electrochimica Acta.2015, 180, 129-137.
- Maulidiyah; Ritonga, H.; Salamba, R.; Wibowo, D.; Nurdin, M. International Journal of ChemTech Research.2015, 8, 645-653.
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Applied Catalysis A. 2015, 495, 131-140.
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Energies. 2012, 5, 4952-5001.
- Amano, F.; Ishinaga, E.; Yamakata, A. The Journal of Physical Chemistry C.2013, 117, 22584-22590.
- Huang, P.-H.; Jhan, J.-W.; Cheng, Y.-M.; Cheng, H.-H. The Scientific World Journal. 2014, 2014, 1-8.
- Park, Y.; Kim, W.; Park, H.; Tachikawa, T.; Majima, T.; Choi, W. Applied catalysis B: Environmental.2009,91, 355-361.
- Taziwa, R.; Meyer, E. Advances in Nanoparticles. 2014,3, 54-63.
- Huang, W.-F.; Wu, P.-J.; Hsu, W.-C.; Wu, C.-W.; Liang, K.S.; Lin, M.C. Journal of Theoretical and Computational Chemistry. 2013, 12, 1-15.
- Rahmawati, F.; Wahyuningsih, S.; Handayani, N. Indonesian Journal Chemistry, 2008, 8, 331-336.
- Baba, A.A.; Adekola F.A.; Arodola O.A.; Ibrahim L.; Bale R.B.; Ghosh M.K.; Sheik A.R. Metall. Mater. Eng. 2012, 18, 67-78.

This work is licensed under a Creative Commons Attribution 4.0 International License.









