Pre-Column derivatization Chiral HPLC Method for the separation and quantification of (R,R)-2,8-diazobicyclo [4.3.0]nonane content in (S,S)-2,8-diazobicyclo[4.3.0]nonane, A Key Intermediate of Moxifloxacin Hydrochloride
Bulusu Lakshmi sushma* 1, 2, G. Madhusudhan1, A. Jayashree2, and Koti Reddy Yeruva3
1Inogent Laboratories Pvt.Ltd., 28-A, IDA Nacharam , Hyderabad, 500 076, Telangana, India. 2Centre for chemical sciences & Technology, Institute of Science and Technology, Jawaharlal Nehru Technological University Hyderabad, Kukatpally, Hyderabad 500085, Telangana, India 3Laboratories Limited, API Plant-3, Medak District, Hyderabad, Telangana, India. Corresponding Author Email: sushma.bulusu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310444
Article Received on :
Article Accepted on :
Article Published : 04 Dec 2015
A simple and robust analytical method was developed for the separation and quantification of (R,R)-isomer in (S,S)-2,8-diazobicyclo[4.3.0]nonane, a non chromophoric moiety and key intermediate in the synthesis of Moxifloxacin hydrochloride by using pre-column derivatization reagent 4-Chloro-7-nitrobenzofurazan (NBD Chloride). The derivatization was brought to be optimized at room temperature. The separation was achieved on Chiralpak IC 250 mm × 4.6 mm, 5 µm column with isocratic elution mode with a flow rate of 1.0 mL min-1. The mobile phase was a mixture of methanol, ethanol and diethyl amine in the ratio of 1:1:0.1 (v/v/v). The eluents were monitored at 340 nm using a UV detector.
KEYWORDS:Moxifloxacin hydrochloride; HPLC; Validation; Nonane; Pre-column derivatization; Non chromophoric; Enantiomeric separation
Download this article as:| Copy the following to cite this article: Sushma B. L, Madhusudhan G, Jayashree A, Yeruva K. R. Pre-Column derivatization Chiral HPLC Method for the separation and quantification of (R,R)-2,8-diazobicyclo [4.3.0]nonane content in (S,S)-2,8-diazobicyclo[4.3.0]nonane, A Key Intermediate of Moxifloxacin Hydrochloride. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Sushma B. L, Madhusudhan G, Jayashree A, Yeruva K. R. Pre-Column derivatization Chiral HPLC Method for the separation and quantification of (R,R)-2,8-diazobicyclo [4.3.0]nonane content in (S,S)-2,8-diazobicyclo[4.3.0]nonane, A Key Intermediate of Moxifloxacin Hydrochloride. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12981 |
Introduction
Moxifloxacin (1-Cyclopropyl-7-(S,S)-2,8-diazabicyclo(4.3.0)-non-8-yl-6-fluoro-8 methoxy-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid hydrochloride), is a new fourth generation 8-methoxyfluoroquinolone developed primarily for the treatment of community acquired pneumonia and upper respiratory tract infections. It is active not only against gram-negative pathogens but also against gram-positive cocci aerobic intracellular bacteria, a typical organisms and anaerobic bacteria. One of the widely used fourth generation fluoro quinolone is Moxifloxacin. It has enhanced activity against specific bacteria, such as mycobacterium, and Legionella. Moxifloxacin is designed to treat community-acquired respiratory tract infections, showed better efficacy than ciprofloxacin against S. maltophilia. For preparing the quinolone derivative like Moxifloxacin, enantiomerically pure (S,S)-2,8-diazabicyclo[4.3.0] nonane is required (S,S)-2,8-Diazobicyclo[4.3.0] nonane (Fig-1) is a key intermediate of Moxifloxacin, which was also used for constructing quinolone and naphtyridine derivatives having antibacterial effectiveness [1]. Several HPLC methods have been reported on pre column derivatization techniques , Determination of lisinopril in dosage forms and spiked human plasma through derivatization with 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) followed by spectrophotometry or HPLC with fluorimetric detection[2], ) Pre‑Column derivatization HPLC procedure for the quantitation of aluminium chlorohydrate in antiperspirant creams using quercetin as chromogenic reagent[3], determination of domoic acid in mussels by HPLC with post-column derivatization using 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) and fluorescence detection.[4], Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: a review[5], Pre-column Derivatization RP-HPLC Determination of Amino Acids in Asparagi Radix before and after Heating Process[6] . 4-Chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl) is an activated halide that has been used for the colorimetric determination of some primary and secondary amines [7, 8], for the fluorimetric assay of some amines and amino acids [9, 10] and also for pre-column derivatization of non responding compounds such as amino acids to enable their fluorescence or UV detection in liquid chromatography [10], chiral separation liquid chromatography, derivatization techniques[11-12].
No analytical methods are reported for the quantification of (R,R)-isomer of (S,S)-2,8-diazabicyclo[4.3.0] nonane by using chemical derivatization technique by HPLC (High performance liquid chromatography). To the best of our knowledge there is no chiral LC approach in the literature for the chiral purity determination of (S,S)-2,8-diazabicyclo[4.3.0] nonane. Our aim was to develop and validate a new non aqueous chemical derivatization methodology for determination of undesired (R,R)- 2,8-diazabicyclo[4.3.0] nonane. It is validated analytical method validation in terms of major parameters such as system suitability, precision, accuracy, linearity, robustness ,limit of quantification and limit of detection are provided to be adequate for routine chiral analysis in quality control. Identification limits must be established for other isomer of (S,S)-2,8-diazabicyclo[4.3.0] nonane in accordance to ICH guidelines [13].
Experimental
Materials and Methods
Samples of (S,S)-2,8-diazabicyclo[4.3.0] nonane and it’s enantiomer obtained from our Research & Development laboratory utilizing for the purpose. HPLC grade methanol, ethanol and diethyl amine were obtained from Merck, Mumbai, India.
A Waters Model Alliance 2695-separation module (Waters corporation, Milford, MA, USA) equipped with a waters 2998-photo diode array detector was used. Data was processed through Waters empower software. Isocratic analytical method with Chiral Pak IC, 250 x 4.6mm i.d., 5mm particle size (Daicel Chemical Industries, Tokyo, Japan) with a mobile phase consisting of methanol, ethanol and diethyl amine in the ratio of 50:50:0.1 v/v/v. Column temperature was maintained at 35°C. Flow rate was kept at 1.0 mL min-1 and the column eluent was monitored at 340 nm for 40 minutes.
Preparation of Stock and Standard Solutions
Stock solutions were prepared by dissolving 0.5 mg mL-1 of racemic mixture, 0.5 mg mL-1 of (R,R)-2,8-diazabicyclo[4.3.0]nonane and (S,S)-2,8-diazabicyclo[4.3.0]nonane precisely weighed in respective 10 mL volumetric flasks, dissolved in 5 mL diluent and made up to the mark with diluent.
![Fig. 1. Chemical structures of (S,S)-2,8-Diazabicyclo[4.3.0] nonane and its (R,R)-isomer](http://www.orientjchem.org/wp-content/uploads/2015/12/Vol31_No4_Pre_Bulu_Fig1-150x150.jpg) |
Figure 1: Chemical structures of (S,S)-2,8-Diazabicyclo[4.3.0] nonane and its (R,R)-isomer Click here to View figure |
Preparation of Diluent
100 mg of 4-Chloro-7-nitrobenzofurazan (NBD-chloride) was transferred into 100 mL volumetric flask dissolved and dilute to volume with acetonitrile. Chemical structure of NBD Chloride shown in Fig-2.
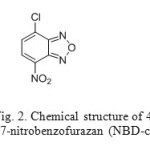 |
Figure 2: Chemical structure of 4-Chloro-7-nitrobenzofurazan (NBD-chloride) Click here to View figure |
Results and Discussion
Polysaccharide based columns such as Chiralpak AD-H, Chiral cel OD, OD-H, OJ-H, Chiralpak IA and Chiralpak IC were tried for separation of enantiomers with different mobile phase combinations of 2-propanol, n-propanol, ethanol and methanol as organic modifiers in n-hexane. Diethylamine (0.1 %) was added to reduce the tailing of peaks. The chiral recognition mechanism on these CSPs is generally due to the formation of solute–CSP complexes [14, 15]. Chiralpak IA column has shown little separation without baseline resolution for the nonane enantiomers. With methanol (4 %) and ethanol (6 %), it showed a resolution < 1.0. As Chiralpak IC column has shown better results when compared to Chiralpak IA column for the separation of (R,R)-isomer & (S,S)-isomer. Hence, Chiralpak IC column was chosen for further development. When ethanol and methanol in the ratio of 1:1 v/v was used as mobile phase for the enantiomers were separated on Chiralpak IC column was more favorable to the chiral interaction between the solutes and the CSP. Finally, 0.1 % DEA was chosen as organic modifier for separation of nonane enantiomers. As the concentration of ethanol in the mobile phase was decreased, retention factors as well as resolutions were increased. As a compromise for higher resolution and lower retention, 50% ethanol was chosen for optimum separation.
Thus a mobile phase containing methanol: ethanol: DEA (50:50:0.1 v/v/v) was chosen for separation of enantiomers on Chiralpak IC column. The flow rate was kept at 1.0 mL min-1 throughout the analysis. The chromatographic separation of ( (S,S) and (R,R)- enantiomers of 2,8-Diazabicyclo[4.3.0] nonane in the optimized conditions using UV at 340 nm. The method was validated in terms of accuracy, precision and linearity as per ICH guidelines.[11]
Method Validation
System Suitability
The solution of racemic (R,S)-2,8-diazabicyclo[4.3.0]nonane (0.5 mg mL-1) prepared in the diluent was used for system suitability studies. The Chiralpak IC column was equilibrated for 30 min under the optimized conditions and three replicate injections were made. The system was deemed to be suitable if resolution between the two nonane isomers is not less than 1.5. The number of theoretical plates for (R,R)-2,8-diazabicyclo[4.3.0]nonane and (S,S)-2,8-diazabicyclo [4.3.0]nonane were 7860 and 6900, tailing factors were 1.18 and 1.24 and the resolution is 1.9 respectively. System suitability chromatogram shown in Fig-3.
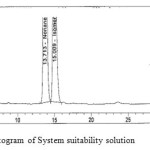 |
Figure 3: Typical chromatogram of System suitability solution Click here to View figure |
Precision
Prepared six individual sample solutions spiked with(R,R)-2,8-diazabicyclo[4.3.0]nonane enantiomer at specification level and injected in to the chromatograph. Calculated % RSD for the areas and % (R,R)-2,8-diazabicyclo[4.3.0] nonane enantiomer. The %RSD for the area was <0.4% and for (R,R)-2,8-diazabicyclo[4.3.0] nonane enantiomer content is <0.45%.
Linearity
Calibration graphs were drawn in the range of LOQ to 150% of specification level for (R,R)-2,8-diazabicyclo[4.3.0] nonane enantiomer. The calibration curves is linear with correlation co-efficient ≥ 0.9999.
Accuracy
The accuracy of the method was determined by spiking (R,R)-2,8-diazabicyclo[4.3.0]nonane solution at three levels in the range 50%–150% with respective to specification level and analyzing the each solution in triplicate. The percentage recoveries were between 90.4 % and 92.4 %. results are shown in Table 1.
Table 1: Accuracy/Recovery results
|
Preparation |
At 50 % level |
At 100 % level |
At 150 % level |
|
1 |
91.2 |
92.0 |
92.4 |
|
2 |
90.4 |
91.6 |
92.2 |
|
3 |
90.4 |
92 |
92.0 |
|
Average |
90.6 |
91.8 |
92.2 |
Robustness
Robustness of the method was checked by making small deliberate changes in the operating conditions. Variation of ethanol and methanol affect the resolution and retention time were changed. The effect of temperature was studied by analyzing sample at 35 ±5°C. The resolution remained still more than 1.5. The effect of flow rate was studied by analyzing the samples with 0.8 mL min-1 and 1.2 mL min-1 flow rates. In both the cases resolution was observed more than 1.5.
LOD and LOQ
Limits of detection (LOD) and quantification (LOQ) were calculated using signal/noise (S/N) ratio method. LOD was taken as the concentration of the analyte where S/N was 3 and it was found to be 0.015% (R,R)-2,8-diazabicyclo[4.3.0] nonane respectively. LOQ was taken as the concentration of the analyte where S/N is 10 and it was found to be 0.05% for (R,R)-2,8-diazabicyclo[4.3.0] nonane. The percentage recovery of (R,R)-2,8-diazabicyclo[4.3.0]nonane enantiomer at accuracy at LOQ level was between 98.7% and 103.9% the results are shown in Table 2. LOD and LOQ reference chromatograms shown in Fig-4 and Fig- 5.
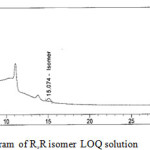 |
Figure 4: Typical chromatogram of R,R isomer LOQ solution Click here to View figure |
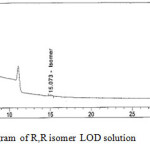 |
Figure 5: Typical chromatogram of R,R isomer LOD solution Click here to View figure |
| Table 2: Limit of detection and Limit of quantification result | |
| Parameter | Result |
| LOD | 0.015% |
| LOQ | 0.05% |
| Precision at LOQ(%RSD) | 3.19 |
| Accuracy at LOQ(n=3) | 101.30% |
Conclusion
Direct resolution of nonane isomers on three different polysaccharide based chiral stationary phases, viz., Chiralpak AD-H,Chiral cel OD,OD-H,OJ-H, Chiralpak IA and Chiralpak IC was studied. Chiralpak IC shown better resolution compared to Chiralpak AD-H and Chiralpak IA column. Baseline separation with resolution >1.5 was achieved between the two nonane isomers within 40 min. The effect of organic modifiers on resolution and retention of isomers was evaluated and the mobile phase composition was optimized. The method was validated with respect to accuracy, precision, linearity, and robustness as per ICH guidelines. The developed method is quite simple, rapid, sensitive and enantioselective and could be of use for evaluation of chiral purity of (S,S)-2,8-diazabicyclo[4.3.0] nonane during the production of moxifloxacin hydrochloride in pharmaceutical bulk drugs and also this method is capable for quantification of(S,S)-2,8-diazabicyclo[4.3.0] nonane content in moxifloxacin hydrochloride samples.
Acknowledgements
The authors wish to thank the management of M/s Inogent Laboratories Pvt. Ltd. for permitting to carry out the present work. The authors also wish to thank the colleagues of Analytical Research & Development and Process Research & Development department for supporting this work.
References
- Hoogkamp-korstanje jaa, roelofs-willemse j. Journal of antimicrobial chemotherapy,2000; 45:31–39
- El-Emam, A. A.; Hansen, S. H.; Moustafa, M. A.; El-Ashry, S. M.; El-Sherbiny, D. T.; J Pharm Biomed Anal. 2004, 34:35-44
- Kalogria, E.; Varvaresou, A.; Papageorgiou, S.; Protopapa, E.; Tsaknis, I.; Matikas, A.; Panderi, I.; Chromatographia. 2014, 77:1275–1281
- Maroulis, M.; Monemvasios, I.; Vardaka, E.; Rigas, P.; J Chromatography B. 2008, 876: 245–251.
- Ilisz, I.; Berkecz, R.;, Peter, A.; J Pharm Biomed Anal . 2008, 47:1–15.
- Zhihong, Shi.; Hui, Li.; Zhimin, Li.; Junda, Hu.; Hongyi, Zhang.; IERI Procedia. 2013, 5: 351 – 356
- Tan, HSI.; J Pharm Sci.1973 62: 993– 997.
- Schnekenburger, SH.; Analyst. 1992, 117, 87–92. [doi:10.1039/an9921700087]
- Ghosh, P.B.; Whitehouse, M.W.; Biochem J.1968, 108: 155–156.
- Ahnoff, M.; Grundevik, I.; Arfwidsson, A.; Fonselius, J.; Persson, B.; Anal Chem. 1981,53: 485–489.
- Ahnoff , M.;, Einarsson, S.; In:J Lough (ed.) Chiral Liquid Chromatography, 1989 39-80.
- Lingeman, H.; Underberg, W.J.M.;. Chromatographic science series. 1990, 48:193–216.
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (I.C.H.), Q2/R1, Validation of analytical procedures. Text and methodology, 1995.
- Chankvetadze, B.; Yamamoto, C.;Okamoto, Y.; Journal of chromatography A. 2001,922, 127–137.
- Yashima, E.; Journal of chromatography A. 2001, 906: 105–125.

This work is licensed under a Creative Commons Attribution 4.0 International License.









