Optical Scattering and Absorption Characteristics of Silver and Silica/Silver Core/shell Nanoparticles.
Sara Mohammadi Bilankohi
Department of Physics, Payame Noor University, Iran. Corresponding Author Email :sara.mohammadibilankohi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310452
Article Received on :
Article Accepted on :
Article Published : 21 Nov 2015
The optical properties of spherical silver and silica/silver nanoparticles are calculated using classical electrodynamics. The wavelength corresponding to maximum extinction shifts to longer wavelengths as the size of the nanoparticle is increased. The influence of higher-order multipoles is evident for large nanoparticles, making the spectra more complex. When the shell thickness of a core/shell particle is decreased, the plasmon resonance shifts to longer wavelengths. This red shift is accompanied by an increase in peak intensity. A model for core/shell nanoparticles is presented to investigate surface coverage effects. This model can be used to interpret the optical properties during the growth process or to examine the effects of shell defects, uneven growth, and surface roughness. The preliminary results for low surface coverage show an increase in extinction as the number of surface particles is increased. The peak position for the array of surface particles matches the resonant wavelength for an isolated spherical nanoparticle with a radius equal to half the shell, size, as expected for the uncoupled limit.
KEYWORDS:Mie Theory; Nanoparticles; Optical Properties
Download this article as:| Copy the following to cite this article: Bilankohi S. M. Optical Scattering and Absorption Characteristics of Silver and Silica/Silver Core/shell Nanoparticles.. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Bilankohi S. M. Optical Scattering and Absorption Characteristics of Silver and Silica/Silver Core/shell Nanoparticles. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12661 |
Introduction
Particles with dimensions on the order of a billionth of a meter are known as nanoparticles. These particles are often composed silver, or gold. When excited with an electromagnetic field, nanoparticles produce an intense absorption attributed to the collective oscillation of electrons on the particle surface, termed a plasmon resonance. The resonant frequency is highly dependent on particle size, shape, material, and environment. By altering these characteristics, the frequency can be shifted over a wide range of wavelengths, making nanoparticles attractive as functional materials for many applications. Some examples include electronic and optical devices of coinage metals such as copper1. The light scattering from nanoparticle surface due to the oscillatory motion of nanoparticle electrons. These electrons produces radiation. Also, if the light is absorbed by the nanoparticle, the energy of the incident light is converted to another form e.g. heat. The optical properties is strongly dependent on size, shape and composition of the particles and medium of nanoparticles are embedded. By modifying these parameters, we can simulate the some optical properties of nanoparticles like scattering, absorbing and extinction (sum of the absorbed and scattered energy) over a wide range of wavelengths. These optical properties of nanoparticles interesting as functional materials for many applications such as electronic and optical devices2, chemical and biological sensors3, optical energy transport4 and thermal ablation5.
In recent years, more attention has to silver nanoparticles due to the optical properties and its application6. When silver nanoparticles interact with the light, according to the fluctuation of the electron band on nanoparticle surface, a phenomenon called surface plasmon occurs. Silver nanoparticles plasmon modes have a high absorption in the ultraviolet and violet spectral region7. Also, when a silica and silver core/shell nanoparticles is used, a sharp plasmon frequency is observed, that can be reduced by changing the diameter of the core and shell. Recently, the strong photostimulated luminescence is obtained from silver and silver iodide nanoclusters8-10. The main advantage of using spherical geometry of nanoparticles is control of optical properties in a highly predictive state11-15. Also, the optical properties of a material with sphere geometry can be simulated by using the solution of Maxwell’s Equations for arbitrary radius and dielectric constant. Therefore, we uses the Mie theory solution for simulation of the optical properties of silver and core/shell silica/silver nanoparticles and discuss about their optical properties.
Simulation Method
In 1908, Gustav Mie obtained the solution of Maxwell’s equations for spherical particles12. These solutions, showed the extinction spectra of spherical particles with arbitrary size. Researchers are also trying to find the Maxwell’s Equations solution for other shapes like nonspherical particles, Au spheroidal particles and Ag spheroidal particles that it was the result of Mie research14. Bohren and Huffman described the complete conclusion of Mie theory and in this paper, we used it13. According to this theory, the cross section parameter can be calculated. This parameter is a quantity that show the power of the scattered, absorbed or extincted of the incident light waves. Since that the sum of the scattered and absorbed cross section is extincted cross section, we can calculate the absorption cross section.
So, we considered a spherical particle with radius r0 and complex electric permittivity ɛ=n2 embedded in a dielectric medium of permittivity ɛm=nm2. This particle is illuminated by a plane wave of angular frequency. Within the Mie solution, the cross-sections for scattering, extinction and absorption can be expressed by following formulas:
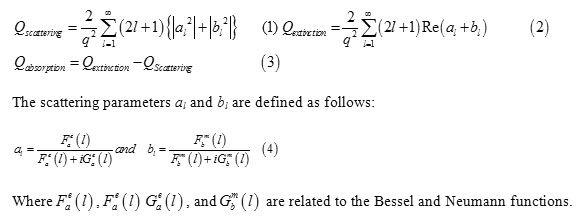
Results and Discussion
The scattering and absorption properties of silver nanoparticles are dependent on the nanoparticle diameter. The extinction cross section area spectra of 10 sizes of silver nanoparticles are displayed in the Figure. 1. The identical mass concentrations for these nanoparticles is 0.05 mg/mL. The silver nanoparticles absorb light and have peaks near 404 nm (Figure.2). All spectra have a high resonance at visible spectrum(400 nm – 700nm) (Table 1). The wavelength corresponding to maximum extinction shifts to longer wavelengths (red shift) as the particle radius increases (Figure.3), while larger particles show increased scattering and have peaks that broaden significantly and shift towards longer wavelengths (known as red-shifting) and generally lies to the near infra-red (Figure.4). High light scattering of the larger particles due to have larger optical cross sections.
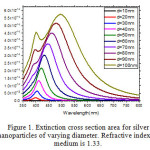 |
Figure 1: Extinction cross section area for silver nanoparticles of varying diameter. Refractive index of medium is 1.33. |
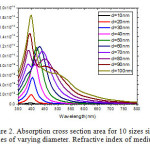 |
Figure 2: Absorption cross section area for 10 sizes silver nanoparticles of varying diameter. Refractive index of medium is 1.33. Click here to View figure |
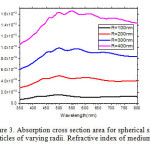 |
Figure 3: Absorption cross section area for spherical silver nanoparticles of varying radii. Refractive index of medium is 1.33. Click here to View figure |
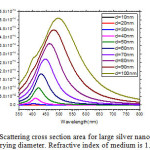 |
Figure 4: Scattering cross section area for large silver nanoparticles of varying diameter. Refractive index of medium is 1.33. Click here to View figure |
The Figure.5 displays the calculated optical spectrum of a 50 nm silver nanoparticles as the local refractive index is increased. Increasing the refractive index from 1 to 1.33.
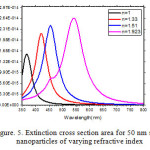 |
Figure 5: Extinction cross section area for 50 nm silver nanoparticles of varying refractive index Click here to View figure |
Table 1: Resonance peak of the optical properties of silver nanoparticles
| Diameter | Scattering Cross Section Peak(nm2) | Wavelength(nm) | Absorption Cross Section Peak(nm2) | Wavelength(nm) | Extinction Cross Section Peak(nm2) | Wavelength(nm) | Atomic Molarity(mmol/L) | Particle Concentration (particle/ml) |
| 10 | 1.06 | 400 | 260 | 395 | 261 | 395 | 0.464 | 9.103×1012 |
| 20 | 104 | 400 | 1.99×103 | 40 | 2.09×103 | 400 | 0.464 | 1.138×1012 |
| 30 | 990 | 404 | 5.58×103 | 404 | 6.57×103 | 404 | 0.464 | 3.372×1011 |
| 40 | 3.96×103 | 409 | 9.43×103 | 409 | 1.34×104 | 409 | 0.464 | 1.422×1011 |
| 50 | 1.02×104 | 422 | 1.18×104 | 418 | 2.17×104 | 418 | 0.464 | 7.283×1010 |
| 60 | 1.84×104 | 431 | 1.19×104 | 427 | 3.04×104 | 431 | 0.464 | 4.214×1010 |
| 70 | 2.71×104 | 449 | 1.10×104 | 440 | 3.79×104 | 445 | 0.464 | 2.65×1010 |
| 80 | 3.62×104 | 463 | 1.11×104 | 454 | 4.50×104 | 458 | 0.464 | 1.778×1010 |
| 90 | 4.42×104 | 481 | 1.47×104 | 395 | 5.16×104 | 476 | 0.464 | 1.249×1010 |
| 100 | 5.11×104 | 499 | 1.85×104 | 400 | 5.75×104 | 494 | 0.464 | 9.103×109 |
Figure. 6 shows the optical properties for silica\silver core\shell nanoparticles with different sizes. The total nanoparticles radius is kept constant at 50 nm. The spectrum with a peak at 498 nm is the extinction from a silver sphere with a radius of 50 nm (no silica core). As the shell thickness is decreased, the peaks shift to the red and become more intense. Increasing the ratio of core radius to total radius causes the peak to shift red. Thinning the shell layer produces a large increase in polarization at the sphere boundary, which yields the more intense extinction peaks. The same general trends are observed when the nanoparticle size is increased.
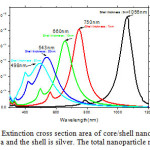 |
Figure 6: Extinction cross section area of core/shell nanoparticles. The core is silica and the shell is silver. The total nanoparticle radius is 50 nm. Click here to View figure |
Conclusions
The optical properties of spherical silver and silica/silver nanoparticles can be tuned by adjusting the physical dimensions. The dielectric properties of the material are extremely important and play a large role in the intensity and placement of the plasmon resonances. As spherical silver and silica/silver nanoparticles get larger, the peaks broaden and shift to longer wavelengths. Higher-order modes also become important, making the extinction spectrum more complex. Core/shell nanoparticles display a red shift and an increase in intensity of extinction as the shell size is decreased. The magnitude of the shift is highly dependent on the shell material. The surface coverage model shows the correct behavior in the low coverage limit. Future work will extend this to high coverages, gleaning knowledge about optical properties of core/shell nanoparticles during the growth process and about the effects of surface defects.
References
- Ghorbani, H. R. Orient J Chem. 2014, 30(4), 1941-194.
- Nandanwar, R.; Singh, P.; Syed, F. F; Haque, F. Z. Orient. J. Chem. 2014, 30(4), 1577-1584.
- Pour, P. G.; Takassi, M. A.; Hamoule, T. Orient. J. Chem. 2014, 30(3),1365-1369.
- Mohammadibilankohi, S.; Ebrahimzadeh, M.; Ghaffary, T.; Zeidiyam, M. Indian Journal of Science and Technology. 2015, 8(9), 27-30.
- Ghaforyan, H.; Ebrahimzadeh, M.; Ghaffary, T.; Rezazadeh, H.; Sokoutjahromi, Z. Chinese Journal of Physics. 2014, 52(1), 233-238.
- Bilankohi, S. M. Orient. J.Chem. 2015, 31(Special Issue), 293-297.
- Saha, K.; Sarit, S.A.; Chaekyu, K.; Xiaoning, L. Chemical Reviews. 2012, 112 (5), 2739-2779.
- Bilankohi, S.M.; Ebrahimzadeh, M.; Ghaffary. T. Indian Journal of Science and Technology. 2015, 8(9), 31-33.
- Soni, S.; Tyagi, H.; Taylor, R.A. Journal of Thermal Biology. 2014, 43 (1), 70-80.
- Fleming, L.A.H.; Tang, G. Optical Materials Express. 2014, 4(5), 969-975.
- Rivero, P.J.; Goicoechea. J.; Urrutia, A.; Matias, I.R. Nanoscale research letters. 2013, 8(1), 1-10.
- Yan, H.; He, L.; Zhao, W.; Li, J.; Xiao, Y.; Yang, R.; Tan, W.; Analytical chemistry. 2014, 86(22), 11440-11450.
- Mie, G. Weinheim, Germany.1908, 25, 377–445.
- Rezazadeh, H.; Ebrahimzadeh, M. Orient. J. Chem. 2013, 29 (2), 469-473.

This work is licensed under a Creative Commons Attribution 4.0 International License.









