Features of Obtaining Silver Nanoparticles in Non-Aqueos Media by Reduction of Silver Trifluoroacetate.
V. N. Glushko, N. Y. Sadovskaya, O. A. Usova, L. I. Blokhina, V. I. Kozhukhov
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances» (FSUE «IREA»), 3, Bogorodskyval, Moscow, 107076, Russia. Corresponding Author Email: tetrazoli@yandex.ru
DOI : http://dx.doi.org/10.13005/ojc/310488
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2015
Possibilityofsilvernanoparticlesynthesisbyreductionofsilvertrifluoroacetateinorganicmedia (ethylene glycol, 2-methoxyethanol, ethyl acetate) was studied. Quercetin, ascorbicacidandpotassium citrate were used asreducing agents.
KEYWORDS:Silver nanoparticles; chemical synthesis; reduction; silver trifluoroacetate; non-aqueous media
Download this article as:| Copy the following to cite this article: Glushko V. N, Sadovskaya N. Y, Usova O. A, Blokhina L. I, Kozhukhov V. I. Features of Obtaining Silver Nanoparticles in Non-Aqueos Media by Reduction of Silver Trifluoroacetate. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Glushko V. N, Sadovskaya N. Y, Usova O. A, Blokhina L. I, Kozhukhov V. I. Features of Obtaining Silver Nanoparticles in Non-Aqueos Media by Reduction of Silver Trifluoroacetate. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12044 |
Introduction
Recently, a large number of studies on the use of the composite materials containing metal nanoparticles have appeared in various areas of practical human activity.
One of the main uses of silver nanoparticles is to create materials with antimicrobial properties for the production of materials and equipment used in health care, children’s and other institutions, where it is important to provide maintenance of antiseptics [1]. Silver nanoparticles are effectively used for the manufacture of filters that are able to neutralize pathogens contained in the water,to create special fabrics, paints and varnishes with antiseptic properties [2]. Creating composite materials with silver nanoparticles results in obtaining fabrics with high antifungal activity. Polymers modified with silver nanoparticles are used to create implants and new generation prosthesis [3].
Theoretical Analisys
Currently, various methods of silver nanoparticle synthesis are conventionally divided into chemical, biochemical and a method with physical influence on the system.
In case of chemical synthesis of silver nanoparticles, as reducing agents are used inorganic and organic compounds. Sodium borohydride[4, 5] and hydrazine[6; 7; 8]are of ten used among inorganic reducing agents. More often flavonoids [9], trisodiumcitrate [1], tinacetate [10],and less often aldehydes, acids, and sugars [11] are used among organic reducing agents. To increase the stability of nano-sized state, additional components– stabilizers are introduced into the reaction medium. They are usually used as surfactants [12]. Stabilization also can be carried out by a reducing agent, such as sodium citrate.Amedium, in which the reduction process proceeds, may have a stabilizing effect too. Dimethylformamide and ethylene glycol, for example, are acting this way. Using salts of acids with along chain (e.g., salts of lauric, stearic, oleic acid) for the synthesis of silver nanoparticles, can also lead to additional stabilization due to the adsorption of long hydrocarbon chains on the nanoparticle surface [13]. Nanoparticles passivated in such way are stable under normal conditions in solutions and in thin films.Growing nanoparticles in the polymer matrix is one of the effective ways of stabilization [14-16].
Silver reduction can be carried out in aqueous [17, 18], organic [19], aqueous-organic [20, 21] media. Among all currently known methods of synthesis, the most common are – the synthesis in water or in reverse micellar solutions. But in order to introduce silver into paint or polymer materials, which have hydrophobic surface, a dispersion of silver must be obtained in organic solvent. There fore, the purpose of work was to study the possibility of synthesis and evaluation of the stability of silver nanoparticles in an organic medium, namely among ethylene glycol, 2-methoxyethanol and ethyl acetate. Trifluoroacetate was synthesized and used as the initial compound for the synthesis of silver nanoparticles. Ethyleneglycoland 2-methoxyethanol were used as non-aqueous media for nanoparticle stabilization and for providing their stability in time. Quercetin, ascorbicacid and potassium citrate were used as reducing agents.The chosen reducing agents possess sufficient solubility in organic solvents and a high reducing ability.
Ascorbicacid – lactone, strongre ducingagent: its enolgroups are easily oxidized toke to groups
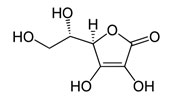
Gamma-lactone 2, 3-dehydro-L-gulonic acid
Quercetin –a natural flavonoid, frequently used as the reducing agent in the synthesis of silver nanoparticles.
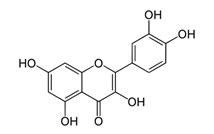
3,3’,4’,5,7 – pentahydroxyflavone Potassium citrate K3C6H5O7
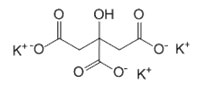
During the obtaining of nanoparticles by citrate method,citrateion acts as a reducing agent and a stabilizer when dissolved in water of trisubstituted potassium salt of citric acid. During the heating of the solution and oxidation of the citrate ion acetone dicarboxylic and itaconic acid are formed.They have the ability to adsorb on the surface of the particles and to control their growth.
Experimental Part
For silver nanoparticle synthesis the following reagents were used:
Silver nitrate – 99.9%, trifluoroacetic acid – 99%, ascorbic acid, quercetin – 95%, sodium citrate, ethyl acetate, ethylene glycol,ethylene glycol methyl ether.
Silver trifluoroacetate synthesis
2.5 g (0,0147 M) AgNO3was dissolved in 20 cm3 of water in a 100 ml flask, then solution of 1.715 g (0,0162 M) sodium carbonate in 30 cm3 of water was added drop wise slowly at room temperature to the AgNO3 solution, stirred for 30 minutes, the precipitate was filtered off, washed with water and dried in a vacuum-desiccator. Trifluoroacetic acid was poured to the residue and the solution was stirred with a magnetic stirrer, rotary evaporated and dried under vacuum. The yield is 2.3 g of silver trifluoroacetate.
Found: С-10,859 %; Obtained: С-11,105 %.
Silver nanoparticle synthesis
Method 1
3 ml 10-3 Mquercetin in ethyleneglycol solution was added to 15 ml 10-3 M silver trifluoroacetate solution in ethyleneglycol, stirred with heating to 70 ºС. A yellowish-brown dispersion is obtained.
Method 2
10 ml 10-3Mascorbicacid in ethyleneglycol solution was added to 20 ml 10-3 M silver trifluoroacetate in ethylene glycol solution, stirred at normal conditions. A yellowish-orange dispersion is obtained.
Method 3
1 ml 10-2M potassium citrate in ethyleneglycol solution was added to 10 ml 10-3M silver trifluoroacetate in ethyleneglycol, stirred with heating to 70 ºС with a magnetic stirrer. Alight yellow dispersion is obtained.
Method 4
3 ml 10-3Mquercetin in methylether solution was added to 15 ml 10-3Msilver trifluoroacetate in ethyleneglyco lsolution, stirred with heating to 75 ºС. Dispersion of orange-red color is obtained.
Method 5
5 ml 10-2Mquercetin in ethyleneglycol methylether solution was added to 25 ml 10-3M silver trifluoroacetate in ethylacetate solution and stirred. Dispersion of orange-red color is obtained.
Method 6
5 ml 10-2Mquercetin in ethyleneglycol methylether solution was added to 25 ml 10-3M silver trifluoroacetate in ethylene glycol methyl ether solution and stirred. Dispersion of orange-red color is obtained.
Elemental analysis was carried out onСНNS-analyzerEurovector «EuroEA 3000».
The absorption spectrum in the UV and visible range of 190 to 1100 nm is recorded using a spectrophotometer SPECORD 250 PLUS.
Results and Disscussions
The most accessible and informative method of identification to study the synthesis of silvernanoparticles is the analysis of the absorptions pectra of the obtained dispersions of nanoparticles with character is ticpl as monresonance peaks that are in the visible wave length range. Tomonitor the completeness of the process of synthesis it is important to observe the changes in the peaks of reducing agents. The absorption spectra of reducing agents, which have been used by us in the synthesis of silver nanoparticles, are presented on the figure 1. Quercetin peaks have the irmaxima at 257,374 nm (curve 1), ascorbic acid – 267 nm (curve 2) and potassium citrate (curve 3) – less than 220 nm, peaks of all reducing agents are in the UV range.
In order to control the process of for mation of silver nanoparticles, the usage of potassium citrate and as corbicacid as reducing agents is more preferably, as their peaks are narrower and are located in shorter wave lengths than quercetin peaks, there by eliminating potential over lapping of silvernanoparticles plasm on peaks.
During the reduction of silver trifluoroacetate in ethylene glycol by quercetin, a stable for several weeks yellowish-brown dispersion is formed (method 1).
By increasing the mixing time (figure 2), followed by heating, the emergence with subsequent increase in the intensity of the long-wave wing of 430-830 nm can be observed. Also, with increased synthesis time, the in tensity of quercetin peaks (258,374 nm)decreases; this indicates gradual silver reduction and consumption of reducing agent. Simultaneously, the increase in the intensity of the absorption band with maximum of 298 nm, which is associated with the formation of oxocomplex of quercetin, the mechanism of which is described in [12], occurs. Nostrongly expressed plasm on peak of silver nanoparticles during the reduction by quercetin was found, however, together with the appearance of long wave wing, and with consumption of the reducing agent, it may be concluded that formation of nanosized silver with a broad size distribution occurs.
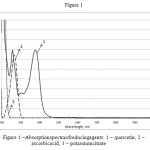 |
Figure 1: Absorption spectra of reducing agents: 1– quercetin, 2 – ascorbicacid, 3 – potassium citrate |
During the reduction of silver trifluoroacetate in ethyleneglycol by as corbicacid, a stable for three weeks dispersion of yellowish-orange silver nanoparticles is formed (method 2).
After mixing solutions of precursor and the reducing agent, the absorptions pectrum in UV and visible region showed the appearance of the plasmon peak (419 nm) of the resulting silver nanoparticles. The increase of the absorption maximum at a wave length of 419 n mindicates an increment of nanoparticle concentration in the solution with continued stirring.
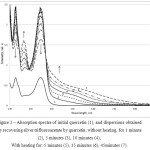 |
Figure 2: Absorption spectra of initial quercetin (1), and dispersions obtained by recovering silver trifluoroacetate by quercetin, without heating, for 1 minute (2), 5 minutes (3), 10 minutes (4),With heating for: 5 minutes (5), 15 minutes (6), 45minutes (7). |
During the reduction of silver trifluoroacetate in ethyleneglycol by potassium citrate, as table for several days dispersion of light-yellow silver nanoparticles is formed (method 3). In this case, the reduction doesn’t occur with out raising the temperature, where as the absorption spectrum of silver nanoparticles (figure 4)has a maximum absorption of 437 nm at 50 ºСafter 5 minutes. The intensity of the absorption band increases with increased up to 30 minutes exposure time with an elevated temperature.
 |
Figure 3: Absorption spectra of initial ascorbi cacid (1),and dispersions obtained by recovering of silver trifluoroacetate by ascorbicacid right after the mixing (2), at 1 minute reaction time (3), at 5 minutes (4), at 10 minutes (5), at 30 minutes (6). Click here to View figure |
The studies revealed that the use of ascorbicacid as a reducing agent in ethyleneglycol medium results in instant aneous silver nanoparticle formation, and this dispersion is s table for three weeks. The most stable dispersion is obtained during the reduction by quercetin; however, silver nanoparticles are formed only at evaluated temperature, like in case with potassium citrate.
To study the in fluence of the nature of the solven ton the silverion reduction, nanoparticle dispersions were obtained using methods 4-6.
Electronic absorption spectra of stable dispersion s were used to control the process of the synthesis.
The dependence of the optical density of the wave length for nanoparticle dispersions, obtained by interaction of silver trifluoroacetate with quercetin, in the following solvents, is presented on the figure 5:
a) methyl ether ethylene glycol (method 4),
b)ethylacetate – methyl ether ethylene glycol system(method 5),
c) ethylene glycol – methyl ether ethylene glycol (method 6).
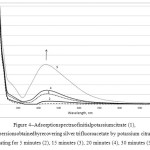 |
Figure 4: Adsorption spectra of initial potassium mcitrate (1), and dispersions obtained by recovering silver trifluoroacetate by potassium citrate with heating for 5 minutes (2), 15 minutes (3), 20 minutes (4), 30 minutes (5). |
During the reduction of silver trifluoroacetate in methyle there thyleneglycol by quercetin, a red-oranged ispersion is formed. There is an absorption maximum at 508 nm in the UV range (curve 1). When ethylene glycol is introduced in the system, the plasmon peak makes a hypsohchromic shift and has a maximum of 427 nm (curve 3). A red-orange dispersion is formed during the reduction of silver in ethyl acetate-methyl ethyl ethylene glycol system. Absorption peak isat 519 nm (curve 2). Presumably, introduction of ethylacetate in to the system favors the formation of nanoparticles of larger sizes than during reduction in the medium of only methyl ether ethylene glycol. That means that the size of nanoparticle scan be varied by changing the solvent, which is valid for silver trifluoroacetate – quercetin system.
 |
Figure 5: Adsorption spectra for nanoparticles, obtained by there action between silver trifluoroacetate with quercetin: in methyle the rehyleneglycol(1), in ethyl acetate – methyl ether ethylene glycol system (2), in ethylene glycol – methyl ether ethylene glycol system (3). Click here to View figure |
Colclusion
In the course of studies it is found, that the use of ascorbicacid as a reducing agent results in instantaneous formation of silver nanoparticles in ethyleneglycol system, and this dispersion is stable for three weeks. The most stabled is persion is obtained by reduction with quercetin; however, nanoparticles don’t form without heating, like in case with potassium citrate. The nature of the solvent has a great influence on the silver trifluoroacetate reduction by quercetin.
Nanoparticles of greater size are formed when ethylacetate is introduced in to the system than in case if reduction is carried out only in the methyle the rethylenglycole medium.
Acknowledgment
Applied researches are carried out with financial support of the state by the Russia Ministryof Education and Science under Grant Agreement No.14.576.21.0024 of June 27, 2014. (Unique identifier for Applied Scientific Researches (project)RFMEFI57614X0024).
References:
- Domènech,B.;Muñoz,M.;Muraviev,D. N.; Macanás, J. Formatex.2013, 1,630-640.
- Revina,A.A.;Egorova,E.M. Chem. Ind.2001, 4, 28-32.
- Kalinichenko, V. S.;PatentRU 2437645.2011.
- Bohren, C. F.;Huffman, D. R. New York, (1983).
- Pugachev,A.D.;Kolesnikov,V.A. XI Inter.Videoconf.2013, 5.
- Ullberg,Z.R.;Podolskaya,V.I.;Voitenko, E. Y.;Grishechenko, N.I.;Yakybenko, L.N.Colloid Mag.2010,72, 70.
- Kim, S.H.; Choi, B.S.; Kang, K.; Choi, Y.S; Yang, S.I. Alloys Comp. 2007,433, 261.
- Gomez, S.;Philippot, K.;Colliere, V.;Chaudret, B.;Senocq, F.;Lecante, P. Chem. Comm. 2000,19, 1945.
- Larina,O.V.;Smagin,V.P. Proc. Acad. Sci.Сhem.2013, 186-189.
- Shankar, R.; Groven, L.;Amert, A.; Whites, K.;Kellar, J.J. Mat. Chem.2011,21, 10871-10877.
- Vishnjakova, E.A.;Saykova, S. V.;Zharkov, S. M.;Likhatsky, M. N.;Mikhlin, J.L. J. Sib. Fed.Un. Chem.2009,2, 48-55.
- Egorova, E. M.;Revina, A.A.;Rostovshchikova, T.N. Bull.Mosc.Univ. Ser. 2. Chem.2001,42(5), 332-338.
- 13.Abe, K.;Hanada, T.; Yoshida, Y.;Tanigaki, N.;Takiguchi, H.;Nagasawa, H.;Nakamoto, M.;Yamaguchi, T.; Yase, K. Thin Sol. Films.1998, 524–527.
- Seljutin, G. E.;Gavrilov, Y. Y.;Voskresenskaya, E. N.;Zakharov, V.A. Chem.Sust. Dev.2010,18,375-388.
- 15.Prozorova, G.F.;Corjova, S.A.;Konkova, T.V.;Ermakova, T.G.;Pozdnyakov, A.S.;Sukhov, B.G.;Arsent’ev, K.Y.;Likhoshvai, E. V.;Trofimov, B.A.Rep. Acad. Sci.2011,437, 1,50–52.
- 16.Gus’kov, V.Y.;Kudasheva, F.H.;Sukhareva, D.A. Sorption and chromatographic processes.2012,12(4), 568-571.
- 17.Kumarn,M.;Reddy,G.;Physica E.2010,42, 1940.
- 18.Hosseini, S. J.; Aghaie H.; Ghaedi M. Orient. J. Chem., 2014, 30(4), 1883.
- 19.Wani, I.A.;Khatoon, S.;Ganguly, A.; Ahmed, J.;Ganguli, A.K.; Ahmad, T. Mater. Res. Bull., 2010,1033, 45.
- Rivas, L.; Sanchez-Cortes, S.;Garcna-Ramos, J.V.;Morcillo, G. Langmuir.2001,17, 5774.
- Revina,A. A.; Busev,S. A.;Belus S. K. ;Glushko,V. N.; SadovskayaN. Y.Orient. J. Chem.,2015, 31(2), 629

This work is licensed under a Creative Commons Attribution 4.0 International License.









