Eluent Influences on Synthesis of Fe(III)-imprinted Polyeugenol using Polyethylene Glycol Diglycidilether (PEGDE) as Cross-linking Agent and its application as Fe(III) sorbent
Muhammad Cholid Djunaidi1,2,*, Dwi Siswanta2 and Jumina2
1Departement of Chemistry , Science and Mathematics Faculty, Diponegoro University of Indonesia 2Departement of Chemistry,Science and Mathematics Faculty Gadjah Mada University of Indonesia Corresponce Author Email: abi_fifa@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310447
Article Received on :
Article Accepted on :
Article Published : 08 Dec 2015
A research on the effect of eluent to remove template and soaking time on the performance of imprinted ionic polymer (IIP) Fe has been carried out. The eluents were HCl, HNO3, and EDTA. The best eluent was used to determine the adsorption selectivity of IIP to Fe (III) compared to Cd (II), Cr (III) and Pb (II). The results showed that the more successful of template removal the better performance of IIP Fe. Removal of Fe(III) was optimum using HNO3 1M as eluent for 24 hours. Selectivity of IIP towards Cd(II) is better than Cr (III) nor to Pb (II).
KEYWORDS:IIP Fe; acid/eluent removal; selectivity
Download this article as:| Copy the following to cite this article: Djunaidi M. C, Siswanta D, Jumina. Eluent Influences on Synthesis of Fe(III)-imprinted Polyeugenol using Polyethylene Glycol Diglycidilether (PEGDE) as Cross-linking Agent and its application as Fe(III) sorbent. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Djunaidi M. C, Siswanta D, Jumina. Eluent Influences on Synthesis of Fe(III)-imprinted Polyeugenol using Polyethylene Glycol Diglycidilether (PEGDE) as Cross-linking Agent and its application as Fe(III) sorbent. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13144 |
Introduction
Iron is an essential mineral for many application. However, its presence in drinking water cause problems, such as discoloration, giving metallic taste, odor, turbidity and give stains on laundry and plumbing. Iron oxides formed in the tank (reservoir) from oxidation of dissolved iron aeral, also evokes the growth of microorganisms in the water so that WHO limit 0.3 ppm of Iron in drinking water [1].
Conventional methods for separating iron from the solution of which hydroxide precipitation, filtration, electrocoagulation, and ion exchange techniques. Adsorption is one of the physicochemical processes that occur at the interface of solid-phase and solid-gas, has become an effective and economical method with great potential for separation, recovery and recycling of heavy metals from waste water [1].
One of the recently developed techniques for the preparation of highly effevtive adsorbent is the ionic-selective imprinting technique, where the specific recogniton capability is provided to the host molecules by addition and subsequent extraction of template molecules/ions. Recently, the heavy metal ions imprinting adsorbent, for its excellent selective separation performance for trace heavy metals, has become more concerned [2,3].
Adsorption of Fe(III) on the printed surface has been reported previously. Chang et al (2007)[4] make printed sorbent Fe (III) silica gel functionalized with amino surface mold method. Owens et al. (2005) [5] make cation exchange resin mold Fe (III) for the selective adsorption of Fe (III). Capacity of Fe (III) to the resin with a small cross-linker agent was 0.0414 mg/g, whereas if a large cross-linker agent capacity doubled to 0.086 mg/g, but selectivity will be dropped [5]. Fan et al. (2012) made a silica gel sorbent functionalised with thiocyanate for selective adsorption of Fe (III) as imprinted. The results increase the adsorption capacity of 9.58 mg/g (NIP) to 20.1 mg/g (IIP) [1].
One interesting study is the use of biomaterials as adsorbent for heavy metal waste [6,7]. Biomaterials are very important because it is cheap, biodegradable and also biocompatible. They can be prepared from a number of different agricultural waste [8], corn husk, bagase, rice husk, lignin, microbial biomass, chitosan [9,10], husk of green gram (phaseolusaureus) seed [11]and eugenol.
Eugenol is one of native Indonesian natural products and has many functions, including for the separation of metal ions. Some examples are the conversion of eugenol into polyeugeniloxyacetate was used for the separation of heavy metal mixtures by solvent extraction method [12]; and its conversion into eugenoxy acetate for the separation of Cr(III) using bulk liquid membrane (BLM) method [13]. Eugenol polymer, polyeugenol, has been used as a BLM carrier with the order of Cr(III) Fe(III)>Ni(II) Zn>Cd (hard medium>soft) [14]. In the present study, eugenol polymer, polyeugenol, is used as functional polymers to prepare Fe(III) imprinted adsorbents.
Eluents have important functionto release template in order to create a specific hole cavity in IIP. The more specific cavity, the more specific adsorption occurs/selective. The objective of this works was investigate influence of eluents to removal of template Fe(III) to explore the potential of ionic imprinted Fe(III) for adsorption Fe(III).
Experimental Section
Materials
Eugenol, BF3O(C2H5)2and PEGDE (Polyethyleneglycoldiglycidyl ether) purchased from SIGMA-Aldrich, while other reagents were purchased from E Merck, Germany: anhydrate Na2SO4, fuming HNO3, NaOH, HCl 37%, Na2EDTA, Pb(NO3)2 standard solution (1000 mg/L), Fe(NO3)3, Cd(NO3)2 , standard solution (1000 mg/L), Cr(NO3)2standard solution (1000 mg/L). Methanol, chloroform, and demineralized water purchased from Bratachem.
Instrumentations
The instruments used in this study were Atomic Absorption Spectrophotometer (Perkin Elmer), FTIR Spectrophotometer (Shimadzu 8201PC), XRD (Shimadzu XRD-8000), SEM EDX (JSM 6380 LA), and analytical balance (Mettler Tolendo AB54-S).
Procedure
Synthesis of Polymers
Polyeugenol
Eugenol (5.8 g) was put in a 3-neck flask, then 0.25 mL of boron trifluoride diethyl ether, BF3O(C2H5)2 was added as catalyst. The addition was done 4 times every hour while stirring with magnetic stirrer at room temperature. The occurrence of a reaction can be characterized by the color change of the solution into red. After the last addition of the catalyst, the polymerization was allowed to continue up to 12-16 hours, after which 1 mL of methanol was added to stop the reaction. The gel produced was dissolved in chloroform and put into a separating funnel and then washed repeatedly with distilled water until neutral. The organic layer was transferred into a 50 mL erlenmeyer flask and added with anhydrous Na2SO4. The liquid was separated by decantation. Afterwards, the solvent was evaporated in rotary evaporator at 40 °C. The residue obtained was further dried in the desiccator, and was subsequently weighed and characterized using NMR and GPC.
Synthesis of IIP-Fe(III)
Polyeugenol (0.5 g) was stirred with (Fe(III) solution with different concentrations for 24 hours. The product was filtered with a filter paper and subsequently air dried at room temperature. Polyeugenol-Fe(III) produced from this process (0.3 g) was then crosslinked using PEGDE as the crosslinker produce polymer aggregate with a mole ratio of 1:1 by heating for 15 minutes at 80-90 °Cwith 20 mL 1M NaOHas catalyst. The product was then neutralized and dried at 115°C in an oven for 6 hours. The resin produced was further treated with acid for 24 hours to release the Fe(III) ions and form the final product of IIP-Fe(III) adsorbent.
Synthesis NIP
NIP was synthesized using the same procedure as the IIP, but without using Fe(III) at the beginning.
Synthesis NIP-HNO3
The procedure for preparing NIP-HNO3 was similar to that used to prepare NIP, however, at the end of the procedure, the polymer was soaked in 1 M HNO3.
Adsorption experiment
The adsorption studies were carried out using batch method at the desired pH, which was by adding diluted NaOH and HNO3. Adsorbent (50 mg) was contacted with 10 mL of metal ion solution for 24 hours at a constant speed. The mixture was filtered with a fine filter paper and the concentration of metal ion in the filtrate were analyzed using Atomic Absorption Spectrometer (AAS).
Eluent Influence Test
The performance of IIP for the adsorption of Fe(III) was optimized for eluent variables: type, concentration and time soaking of eluents. The eluents used for the experiments were HNO3 (at different concentration), HCl and HCl-EDTA. Finally, the removal of Fe(III) was optimized at different soaking time of 12, 24, 36, 48 and 60 hours using 1 M HNO3.
Adsorption selectivity test
was conducted using binary mixture solutions containing Fe(III)/Cd(II), Fe(III)/Cr (III), Fe(III)/Pb(II). This study was carried out for both IIP and NIP-HNO3.
The study of adsorption selectivity was carried out by adding 0.05 g of adsorbent into each of 10 mL binary-solutions containing Fe(III)/Cd(II), Fe(III)/Cr(III), Fe(III)/Pb(II) using concentration of 50 mg/L for Fe(III) whereis competitive ion were made by comparition concentration of 1:1;1:0.5; 1:0.25 towards concentration of Fe(III) ion. The adsorption experiments were carried out with batch system using a magnetic stirrer at pH 3.
Result and Discussion
Synthesis IIP Fe
The synthesis of IIP-Fe comprises of four stages, starting with the polymerization of eugenol. Eugenol was polymerised using BF3 diethyl ether as catalyst, and resulted polyeugenol was characterized by NMR. In Figure 1, it can be seen that the chemical shift at 4.75 to 5.2 ppm appears in the eugenol spectra as a doublet, which indicates the presence of =CH2 groups; this chemical shift is not visible in polyeugenol spectra. Instead, new spectrum appears at 2.4-3.0 ppm of the polyeugenol spectra, indicating the presence of -CH- groups in the polymer. Another new signal which appears at the chemical shift of 0.7-1.5 ppm is the characteristics of 3 hydrogen atoms of methyl groups (-CH3) in the polymer backbone. So it can be concluded that eugenol polymerization reaction had occurred.
To determine how many n of eugenol molecules exist in the polyeugenol chain, gel permeation chromatography (GPC) analysis was done (Figure 2), which shows that the average Mr of polyeugenol is 1876, or about 11 times the monomer (Mr of eugenol is 164).
The polyeugenol was used for the synthesis IIP. This was done by uploading Fe(III) into the polyeugenol chain, followed by crosslinking using PEGDE and the removal of Fe(III) using HNO3.
The effects of different eluents for template ion removal
Template ion (Fe(III)) removal were done using different acids as eluent, and it is an important parameter controlling the properties of adsorbents for heavy metal ion adsorption, as it relates to the availability of “selective hole” to Fe(III). To determine the type eluent, a series of experiments were carried out under the following conditions: Eluents used were HNO3, HCl and Na2EDTA-HCl, pH of the solution was set to 3, the weight of the adsorbent was 50 mg, the concentration of Fe(III) was set at 50 mg/L, and the concentration of Fe(III) solution used as a template is 100 ppm. Table 1shows that 1 M HNO3 has the maximum adsorption. This is because the removal of Fe(III) was also maximum thus causing maximum formation selective holes . HNO3 1 M had also been used in other previous studies [4,5].
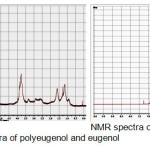 |
Figure 1: NMR spectra of polyeugenol and eugenol Click here to View figure |
Table 1: The difference of % Fe being removed during IIP synthesis and % Fe adsorbed by IIP- Fe(III) using 22 mesh particle size, 0,05 g adsorbent, template ion concentration of 100 mg/L, and 50 mg/L Fe(III) in the adsorption solution.
|
Eluent |
% Fe removed |
% Fe adsorbed |
|
HNO3 0.1 M |
44.59 | 56.29 |
|
HNO3 0.5 M |
48.43 | 76.43 |
|
HNO3 1 M |
53.45 | 95.95 |
|
HCl 1 M |
50.06 | 85.07 |
|
Na2EDTA-HCl |
46.23 | 88.31 |
The effects of contact time (soaking time) with acid/eluent
The contact time (soaking time)of the removal of Fe3+ ions by acid also affects the adsorption efficiency. As can be seen in Table 2, the longer the contact time, the more Fe3+ ions were released, although it also reduced the adsorption capacity, probably due to decomposition of selective sites in IIP by acid. The contact time of 24 hours using HNO3 1 M was used for subsequent experiments in this study.
Tabel 2: Comparison of % Fe being removed from the polymer after treated with 1M HNO3 during IIP synthesis and % Fe(III) being adsorbed by IIP-Fe(III) at 22 mesh
| Soaking time (hours) | % Fe removed | % Fe adsorbed |
|
12 |
64.32 | 96.36 |
|
24 |
65.09 | 97.51 |
|
36 |
69.04 | 97.18 |
|
48 |
70.00 | 95.39 |
|
60 |
75.31 | 58.40 |
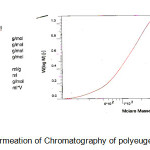 |
Figure 2: Gel Permeation of Chromatography of polyeugenol.
|
As shown in the Fig. 3, the more concentrated acidthe better % adsorption obtained up to a concentration of 3 M. At higher concentrations of 6 M% the adsorption is smaller, probably due to the decomposition of the resin has been used. As seen in the IR spectra below (Fig. 4).
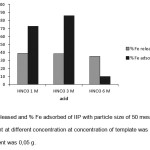 |
Figure 3: % Fe released and % Fe adsorbed of IIP with particle size of 50 mesh with HNO3 acid as eluent at different concentration at concentration of template was 100 mg/L, weight adsorbent was 0,05 g. Click here to View figure |
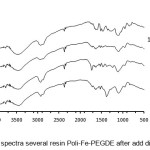 |
Figure 4: IR spectra several resin Poli-Fe-PEGDE after add different acids. |
Table 3: Selectivity Fe(III) 50 mg/L towards Cr(III) 12,5 mg/L.
| Adsorbent |
Kd |
K (Kd Fe/Kd Cr) | |
| Kd Fe | Kd Cr | ||
| IIP | 1,359 | 0,229 | 5,946 |
| NIP HNO3 | 0,175 | 0,271 | 0,645 |
As seemed at Fig 4 above that bigger concentration of acid used, there are new spectraat 1400 cm-1 that shows the formation of new functional groups as result of decomposition. Greatest concentration still possible to use is 1 M, according to the concentration of the acid used in the study.
Table 4: Selectivity Fe(III) 50 mg/L towards Cd(II) 12,5 mg/L.
| Adsorbent |
Kd |
k (Kd Fe/Kd Cd) | |
| Kd Fe | Kd Cd | ||
| IIP | 95159,8 | 0,09 | 1053295 |
| NIP HNO3 | 0,075 | 0,154 | 0,486 |
Table 5: Selectivity Fe(III) 50 mg/L towards Pb(II) 12,5 mg/L.
| Adsorbent |
Kd |
k (Kd Fe/Kd Pb) | |
| Fe | Pb | ||
| IIP | 11,529 | 4,031 | 2,86 |
| NIP HNO3 | 1,515 | 0,429 | 3,535 |
Table 6: Selectivity Fe(III) 50 mg/L towards Cr(III) 25 mg/L.
| Adsorbent |
Kd |
k (Kd Fe/Kd Cr) | |
| Fe | Cr | ||
| IIP | 0,475 | 0,365 | 1,3 |
| NIP HNO3 | 0,1 | 0,128 | 0,791 |
Table 7: Selectivity Fe(III) 50 mg/L towards Cd(II) 25 mg/L.
| Adsorbent |
Kd |
k (Kd Fe/Kd Cd) | |
| Fe | Cd | ||
| IIP | 5,449 | 0,0003 | 16401 |
| NIP HNO3 | 2,451 | 0,042 | 57,993 |
Table 8: Selectivity Fe(III) 50 mg/L towards Pb(II) 25 mg/L.
| Adsorbent |
Kd |
k (Kd Fe/Kd Pb) | |
| Fe | Pb | ||
| IIP | 0,532 | 0,623 | 0,853 |
| NIP HNO3 | 0,209 | 0,101 | 2,071 |
Selectivity
It can be seen from Table 3 until 10 show that the selectivity of IIP towards Fe(III) is better than HNO3-NIP, with the following selectivity sequence: Cd2+>>Cr3+>>Pb2+ or medium>>hard>>soft. The selectivity for Fe(III)/Cd(II) mixture is the highest because Fe(III) can be classified as hard acid, while Cd2+ is a soft acid. The active sites containing hydroxyl groups can also be classified as hard base, thus it is understandable that the selectivity towards Fe(III)/Cd(II) is higher than towards Fe(III)/Cr(III). The adsorption selectivity towards Fe(III)/Pb(II) is the smallest, probably because there are more hydroxyl groups in NIP-HNO3 than in IIP, so that NIP-HNO3 was able to bind with Pb(II) [15]
Table 9: Selectivity Fe(III) 50 mg/L towards Cr(III) 50 mg/L.
| Adsorbent |
Kd |
K (Kd Fe/Kd Cr) | |
| Kd Fe | Kd Cr | ||
| IIP | 0,082 | 0,089 | 0,918 |
| NIP HNO3 | 0,199 | 0,121 | 1,643 |
Table 10: Selectivity Fe(III) 50 mg/L towards Cd(II) 50 mg/L.
| Resin |
Kd |
k (Kd Fe/Kd Cd) | |
| Kd Fe | Kd Cd | ||
| IIP | 10,265 | 0,008 | 121,268 |
| NIP HNO3 | 1,007 | 0,025 | 40,646 |
Table 11: Selectivity Fe(III) 50 mg/L toward Pb(II) 50 mg/L.
| Adsorbent |
Kd |
k (Kd Fe/Kd Pb) | |
| Kd Fe | Kd Pb | ||
| IIP | 0,434 | 0,31 | 1,403 |
| NIP HNO3 | 0,727 | 0,089 | 8,094 |
Conclusion
Based on the experimental results, as more template being removed from the polymer aggregate, higher adsorption of IIP to Fe(III) occurs. The removal of Fe(III) from polymer aggregate was optimum using HNO3 1M as eluent for 24 hours. IIP has better selectivity towards Cd than Cr (III), but not selective to Pb (II).
References
- Fan, H.T;Sun, T., Korean J. Chem. Eng.2012,29, 798-803
- Chen, J.H.; Li, G.P.; Liu, Q.L.; Ni, J.C.; Wu, W.B.; Lin, J.M.,Chem. Eng. J.,2010, 65, 465-473
- Chen, J.H.; Lin, H.; Luo, Z.H.; He, Y.S.; Li, G.P.,Desalination,2011, 277, 265-273
- Chang, X.; Jiang, N.; Zheng, H.; He, Q.; Hu, Z.; Zhai, Y.; Chui, Y.,Talanta,2007,7,38-43
- Owens, G.S.; Southard, G.E.; Houten, K.A.V.; Murray, G.M.,Sep. Sci. Technol., 2005,40, 2205 – 2211
- Sölener, M.; Tunali, S.; özcan A.S.; özcan, A.; Gedikbey, T., Desalination, 2008, 223 (1-3), 308-322
- Guibal, E. Sep. Purif. Technol., 2004, 38 (1), 43–74
- Porselvi, E.; Krishnamoorthy, P. Oriental Journal of Chemistry,2013, 29 (2), 719-723
- Copello, G.J.; Varela, F.; Vivot, M.; Diaz, L.E.Bioresour. Technol., 2008, 99 (14), 6538–6544
- Miretzky, P.; Cirelli, A.F.J. Hazard. Mater.,2009, 167 (1-3), 10–23
- Jirekar, D.B.; Pathan, A.A.; Farooqui, M. Oriental Journal of Chemistry, 2014, 30 (3), 1263-1269
- Harimu, L.; Matsjeh, S.; Siswanta, D.; Santoso, S.J. Indo J. Chem., 2009, 9 (2), 261-266
- Djunaidi, M.C.; Khabibi; Trisna, D.,JSKA, 2010, 13 (2)
- Djunaidi, M.C.; Lusiana, R.A.; Wibowa, P.J.; Siswanta, D.; Jumina, J. Alchemy, 2007, 6, 40-49
- Masykur, A.; Santosa, S.J.; Siswanta, D.; Jumina, Indones. J. Chem.2014,14(2),152-159

This work is licensed under a Creative Commons Attribution 4.0 International License.









