Effect of Chloride ion concentration on Rapid Kinetics of Chlorination of Regioisomers of Nitrophenol by Molecular Chlorine in Aqueous Medium using Rotating Platinum Electrode.
T. M. Sukul, S. L. Bonde*, R. P. Bhadane and V. T. Dangat
Department of Physical Chemistry, Nowrosjee Wadia College, Affiliated to Savitribai Phule Pune University, Pune, India. Corresponding Author Email: sbonde52@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310438
Article Received on :
Article Accepted on :
Article Published : 03 Dec 2015
The rapid kinetics of chlorination of regioisomers of the nitrophenol by molecular chlorine in the presence of chloride ion in aqueous solution has been studied using rotating platinum electrode (RPE). The chloride ion has a catalytic effect on the chlorination reaction. The mechanism suggested explaining the catalytic effect of chloride ion postulates a longer mean life for Cl-Cl dipole induced by the aromatic substrate by electron rearrangement with the chloride ion, which facilitate the electrophilic attack of Cl-Cl on the aromatic substrate in rate determining step. Hence the specific reaction rate varies linearly with the concentration of chloride ion.
KEYWORDS:Rapid Chlorination; Rotating Platinum Electrode; Kinetics
Download this article as:| Copy the following to cite this article: Sukul T. M, Bonde S. L, Bhadane R. P, Dangat V. T. Effect of Chloride ion concentration on Rapid Kinetics of Chlorination of Regioisomers of Nitrophenol by Molecular Chlorine in Aqueous Medium using Rotating Platinum Electrode. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Sukul T. M, Bonde S. L, Bhadane R. P, Dangat V. T. Effect of Chloride ion concentration on Rapid Kinetics of Chlorination of Regioisomers of Nitrophenol by Molecular Chlorine in Aqueous Medium using Rotating Platinum Electrode. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12891 |
Introduction
It was evident that a different mechanism operates for the chlorination in the presence of chloride ions, but the accepted mechanism for the chlorination of aromatic substrate by molecular chlorine involves the polarization of the chlorine molecule by the electron rich site of aromatic substrate and hence followed by the electrophilic attack of the positive end of the dipole1. We have, however, observed that the chloride ion remarkably enhance the specific reaction rate of such chlorination reactions. A similar feature but for some aromatic substrates containing electron releasing group was reported by earlier workers2. As the role of the chloride ion in the actual mechanism of the reaction was unique, no other anion was able to stabilize the dipole and catalyze the reaction in the manner as chloride ion does. The present study has been undertaken to assess the role of chloride ion in such reaction with regioisomers of nitrophenol such as 2-nitrophenol, 3-nitrophenol and 4-nitrophenol.These reactions were too rapid to be studied by conventional technique. Various methods have previously been used to follow the kinetics of rapid reactions. These include potentiometric method3, spectrophotometry4 Voltammetry5 etc. In present study the special technique namely, hydrodynamic voltammetry has been employed. The attainment of the steady diffusion current has been done by rotating the platinum electrode6 at constant speed, the diffusion layer thickness was considerably reduced, thus increasing the sensitivity and hence rate of attainment of equilibrium. The larger current attained with the rotating platinum electrode allows correspondingly smaller current to be measured without loss of accuracy and thus very dilute solution (up to 10-4 M) can be analysed. Among the reactants and products in the reaction only the chlorine was electroreducible at the RPE, hence the kinetics of the reaction has been studied by measuring the diffusion current due to chlorine at various intervals of time from the start of the reaction.
Some rapid halogenations, for instances, have been studied by Rao, Mali and Dangat 7-9 using the RPE. Since such halogenation reactions were often of the second order, their half-lives can be extended by diluting the solutions and the reaction can be carried out using the RPE which can estimate very low concentration of halogens.
The reactions presently studied were the kinetics of chlorination of 2-nitrophenol, 3-nitrophenol and 4-nitrophenol using KNO3 as a supporting electrolyte with different folds of chloride ion in aqueous medium.
The reactions under study can be represented as:-
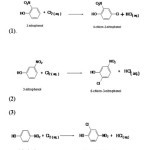 |
Scheme 1 |
The major product of the reaction (1), (2) and (3) were 4-chloro-2-nitrophenol,6-chloro-4-nitrophenol and 2-chloro-4-nitrophenol. Other isomers in each reaction formed in traces10. Due to the rapidity of the reaction the aqueous solutions used in present study are necessarily very dilute and green chemistry principles are inherent in this study11. In the present study the kinetics of the chlorination of 2-nitrophenol, 3-nitrophenol and 4-nitrophenol has been carried out with different folds of chloride ions at the same temperature to find the specific reaction rates of the reactions.
Experimental
The following chemicals have been used in the present study: AR grade samples of 2-nitrophenol, 3-nitrophenol, 4-nitrophenol, potassium nitrate, potassium chloride, sodium thiosulphate, potassium iodide and starch were used to prepare the stock solutions in conductivity water. Chlorine water was prepared from bleaching powder and concentrated hydrochloric acid. It was bubbled through conductivity water to free it from hydrochloric acid & its exact strength was determined by iodometric titration.
Electrodes
The cathode was a platinum electrode fused to a glass tube rotated at 600 rpm with the aid of an ac motor. The anode was a saturated calomel electrode.
The Rotating Platinum Electrode (RPE)
The rotating platinum electrode constructed from a standard ‘mercury seal’. About 5mm of platinum wire protrudes from the wall of length of 6mm inverted t-shaped glass tubing. A pulley & the pair of ball bearing were mounted on the glass tube, having the total length of 32 cm. The ball bearings were fixed rigidly to a stand & the pulley was connected to a synchronous motor. The radius of the pulley was so adjusted that the electrodes rotates at the speed of 600 rpm. The electrical connection was made to the electrode by a stout amalgamated copper wire passing through the tubing to the mercury covering the sealed-in –platinum wire. The inverted T-shape of the electrode facilitates the stirring action.
Calibration of Diffusion current
The RPE and SCE were dipped in 50 cc of 1x 10-1 M potassium nitrate which was the supporting electrolyte. A potential of +0.0 V versus the SCE was applied at RPE. The galvanometer light spot was adjusted to zero deflection on the scale. The potassium nitrate solution was then replaced by 1x 10-3 M chlorine solution. The shunt was adjusted for the deflection of the galvanometer light spot to be around 40 to 50 cm. The shunt was kept constant throughout the experiment. The diffusion current values, in terms of deflection of light spot on the scale, were recorded at various concentrations of chlorine in the range of 0.4 x 10-4 to 2.0 x 10-4 M. The diffusion current in cm was converted into nA by using the sensitivity of the galvanometer.
The plot of deflection observed in cm versus concentration of chlorine was found to be linear as shown in figure. The calibration readings were carried out only after the solutions have attained the thermostat temperature, 28.8⁰C at which kinetic readings were to be observed subsequently.
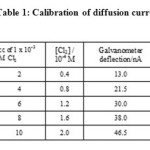 |
Table 1: Calibration of diffusion current |
Potential applied to the RPE versus SCE :0.0V Concentration of KNO3 : 0.1M Temperature : 28.8⁰C
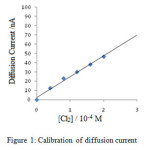 |
Figure 1: Calibration of diffusion current |
Kinetic Measurements
Equimolar solutions of nitrophenol and chlorine containing supporting electrolyte were kept in the thermostat in different containers. After the solutions attained the temperature of the thermostat, these were mixed in the reaction vessel in which the SCE and the RPE were dipped and the stop clock was started. As the reaction proceeds, the galvanometer deflection decreases steadily. This was recorded at every 10 seconds.
The above procedure of calibration and kinetic measurement was repeated thrice for checking the reproducibility of the galvanometer measurements, and these were found to be within the limits ±0.3 cm.
From the observed deflection during the kinetic study, the concentration of the unreacted chlorine (a-x), at various instants were obtained using the calibration curve. A plot of [Cl2 ]-1 versus time t was found to be linear and hence the reaction was of the second order. From the slope of this plot, specific reaction rate k was calculated. The same procedure as given above was used for the kinetic measurement of 3-nitrophenol and 4-nitrophenol.
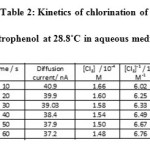 |
Table 2: Kinetics of chlorination of 2-nitrophenol at 28.8˚C in aqueous medium |
Initial concentration of chlorine : 2 x 10-4 M, Initial concentration of 2-nitrophenol: 2 x 10-4 M Temperature : 28.8⁰C
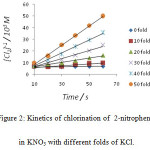 |
Figure 2: Kinetics of chlorination of 2-nitrophenol in KNO3 with different folds of KCl. |
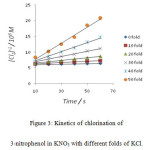 |
Figure 3: Kinetics of chlorination of 3-nitrophenol in KNO3 with different folds of KCl. |
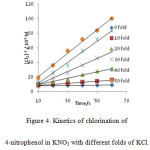 |
Figure 4: Kinetics of chlorination of 4-nitrophenol in KNO3 with different folds of KCl. Click here to View figure |
Results and Discussion
The specific reaction rate determination has been carried out at various folds of KCl at 28.8⁰C. The potassium chloride concentration was varied from 10 to 50 folds. The kinetic measurements have an error of not more than ± 2% in view of the reproducibility of the diffusion current.
It was observed that as the concentration of potassium chloride increases, the specific reaction rate increases. In case of 4–nitrophenol, with zero folds of KCl, the specific reaction rate was 14.17 M-1 s-1 and with the highest fold of KCl, the specific reaction rate was found to be 1529.40 M-1 s-1 . In case of 2-nitrophenol, with zero fold of KCl, the specific reaction rate was 16.67 M-1 s-1 and with the highest folds of KCl, the specific reaction rate was found to be 778.76 M-1 s-1 . In case of 3-nitrophenol, with zero folds of KCl, the specific reaction rate was 10.40 M-1 s-1 and with the highest fold of KCl , the specific reaction rate was found to be 265.48 M-1 s-1 .
When the molecular chlorine was used as a chlorinating agent the polarization of the electrophile, viz. the chlorine molecule takes place due to the inductive effect of substrate ring which was much more prominent in the presence of chloride ions. Hence the positive ends of the dipole in the chlorine molecule Cl+ act as an effective electrophile. In the presence of Cl–, the molecular chlorine was the sole chlorinating reagent and hypohalous acid was known to be a very weak electrophile12and the equilibrium shown below shifted towards left.
![]()
HOCl was known to be a very slow chlorinating agent except in the presence of an acid.
Chlorine in aqueous solution was extensively hydrolyzed to hypochlorous acid, but HOCl was known to be a much slower chlorinating agent than molecular chlorine. Since the hydrolysis equilibrium was established rapidly, the chlorination may be regarded as effectively by molecular chlorine only. The addition of excess of Cl– suppresses this hydrolysis and hence increasein specific reaction rate is observed as the concentration of KCl increases.
It was thus evident that a different mechanism operates for the chlorination in the presence of chloride ions. Since the reaction was of second order, both chlorine and substrate were involved in the rate determining step. Further, since the chloride ion catalyzes the reaction, it was also involved in this reaction. The aromatic substrate with its electron- rich site polarizes the chlorine molecule. In the presence of a large concentration of added chloride ion, for every chlorine molecule reacting with the substrate, there will be many chloride ions in its near vicinity. Here we postulate that the Cl-Cl dipole was stabilized by rearrangement of electrons with any one of these many chloride ions in the vicinity. This facilitates the electrophilic attack of the Cl-Cl on the aromatic substrate in the rate- determining step. By this mechanism the rate of chloride ion catalyze chlorination of the substrate S is Rate = k3 [S] [Cl2] [Cl–]
Since whatever chloride ion taking part in the reaction was regenerated, its concentration remains constant and can be incorporated with k3 so that k’2 = k3 [Cl–]
Hence, Rate = k’2[S] [Cl2]
This explains the second order kinetics of reaction in the presence of chloride ion.
Conclusion
In the present study the specific reaction rate of the regioisomers of nitrophenol has been determined which has been carried out at various folds of chloride ions. It has been observed that the specific reaction rate increases with the increase in the concentration of chloride ions. Since the reaction was of second order, both the chlorine and the substrate were involved in the rate determining step. Since the role of the chloride ion in the mechanism was unique, no other anion was able to stabilize the dipole & catalyze the reaction in a manner as the chloride ion does. Although these reactions were rapid and couldn’t be studied by conventional methods, the RPE has been used to monitor the progress of these reactions to evaluate the specific reaction rates.
References
- March, J; Advanced Organic Chemistry, McGraw-Hill Kogakusha Ltd, Tokyo, 2nd Ed, 1977,.483
- Rao, T.S.; Jukar, R.N.; Dangat, V.T.; Current Science, 1986, 55(10), 483-487
- Patil , D.B ; Thakur , G.J. ; Shende , P.M. ; Oriental Journal of Chemistry, 2011 , 27(1), 197 -202
- Malik , V.S. ; Vannamuthu , I. ; Shafi , S.S. ; Mansoor , S.S. ; Oriental Journal of Chemistry, 2015,. 31(1) , 77-83
- Pourghobadi , Z ; Niazi , A ; Oriental Journal of Chemistry , 2014 ,. 30(1) , 219-227
- Kolthoff, I. M.; Lingane, J.J.; ‘Polarography’, Second Edition, Interscience Publisher, New York, 1952,.1, 421
- Rao,T.S. ; Mali, S.I., Z..Natureforch, 1976, 31a, 1735-36
- Rao T.S.; Mali S.I. and Dangat, V.T.; J. Univ. Poona, SciTech. , 1979, 52, 111-114
- Rao, T.S.; Mali, S.I.; Dangat, V.T.; Tetrahedron, 1978, 34, 205
- Clayden, J.; Greeves, N.; Warren, S.; Organic Chemistry, Second Edition, 483,491
- Pandey, B.; Fulekar, M. H.; Res. J. Chem. sci., 2011, I(1), 111-117
- Cotton, F. A.; Wilkinson; Advanced Inorganic Chemistry, Wiley Eastern 1972, 585,

This work is licensed under a Creative Commons Attribution 4.0 International License.









