Dynamic 1H NMR and Theoretical Study of the Synthesized Phosphorus Ylide Containing of a Carbamate Derivative
Elham Aghdaei, Sayyed Mostafa Habibi-Khorassani*and Mehdi Shahraki
Department of Chemistry, Faculty of Science, University of Sistan and Baluchestan, P. O. Box 98135-674, Zahedan, Iran Corresponding Author Email:smhabibi@chem.usb.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/310475
Article Received on :
Article Accepted on :
Article Published : 05 Dec 2015
A general and practical route has been described for the synthesis of dimethyl-2-[4-bromophenyl phenylcarbamate-N-yl]-3-(triphenylphosphoranylidene) butandioate in excellent yield. The 1H, 13C, and 31P NMR data of synthesized phosphorous ylide 5 indicated two rotational isomers as major Z-5 and minor E-5 forms. Dynamic 1H NMR effect has been investigated around the C=C bonds in the two Z- and E-rotational isomers at variable temperatures. Herein, activation parameters including ΔH≠, ΔG≠, ΔS≠ were also calculated using ab initio and DFT methods at HF/6-31G(d,p) and B3LYP/6-31G(d,p) levels of theory. The experimental and theoretical data indicated that the rotational barrier around the C=C double bond was considerably high and observation of the two rotational isomers was impossible at the high temperatures. The B3LYP level of theory using the 6-31G (d,p) basis set provided more reasonable results in agreement with the experimental data.
KEYWORDS:Dynamic 1H NMR; Ylide; DFT; Rotational barrier; Ab initio
Download this article as:| Copy the following to cite this article: Aghdaei E, Habibi-Khorassani S. M, Shahraki M. Dynamic 1H NMR and Theoretical Study of the Synthesized Phosphorus Ylide Containing of a Carbamate Derivative. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Aghdaei E, Habibi-Khorassani S. M, Shahraki M. Dynamic 1H NMR and Theoretical Study of the Synthesized Phosphorus Ylide Containing of a Carbamate Derivative. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12994 |
Introduction
The development of simple synthetic routes for widely used organic compounds from readily available reagents is one of the major tasks in organic chemistry [1, 2]. Phosphorus ylides are important compounds in organic chemistry because of their application in the synthesis of organic products [3–10], especially in the synthesis of naturally occurring products with biological and pharmacological activities [3-13]. These compounds are most often prepared by treatment a phosphonium salt with a base. The phosphonium salts are prepared from the reaction of a phosphine and an alkyl halide [3-6, 14], though those can be obtained also by the Michael addition of phosphorus nucleophiles to activated olefins [2, 14]. Some of the phosphorus ylides exhibited dynamic 1H NMR effects that affords good information regarding the interchangeable process of rotational isomers that provide important kinetic data [15-19].
In recent years, we have endeavored to expand dynamic 1H NMR studies and theoretical calculations in order to characterizing the synthesized structures and elucidating the detailed kinetics and mechanisms of these reactions [20-24]. Herein, we describe a one-pot four-component synthesis of new stable phosphorus ylide 5 containing a carbamate derivative from reaction between 4-bromophenol 1, phenylisocyanate 2, triphenylphosphine 3 and dimethyl acetylenedicarboxylate (DMAD) 4 in high yield (Fig. 1) along with dynamic 1H NMR effects and theoretical studies around the C-C bond.
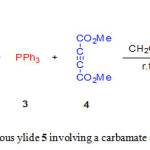 |
Figure 1: Synthesis of stable phosphorous ylide 5 involving a carbamate derivative Click here to View figure |
Experimental
Material, methods and apparatuses used
Melting point and IR spectrum were measured on an Electrothermal 9100 apparatus and on a shimadzu IR-460 spectrometer, respectively. The 1H, 13C, and 31P NMR spectra were recorded on a Bruker DRX-500Avance instrument with CDCl3 as a solvent at 500, 125 and 202 MHz, respectively. Elemental analysis for C, H and N was performed using a Heraeus (Banau, Germany) CHN-O-Rapid analyzer. The mass spectrum was recorded on a shimadzu GC-MS-QP5050 mass spectrometer, operating at an ionization potential of 70 eV. All reagents and solvents were obtained from Merck (Darmastadt, Germany), Across (Geel, Belgium) and Fluka (Buchs, Switzerland), and used without further purification. In order to determine theoretical hindered internal rotational in the rotational interchangeable processes of the two Z- and E-isomers, first their structures were optimized using the ab initio and DFT methods at the HF/6-31G (d,p) and B3LYP/6-31G (d,p) levels of theory by the GAMESS program [25]. Then, relative energy versus dihedral angles was plotted by scanning method at the HF/6-31G (d,p) and B3LYP/6-31G (d,p) levels of theory, and all points in energy profile were fully optimized.
General procedure
First, 4-bromophenol 1 (1 mmol) was added to phenylisocyanate 2 (1 mmol) and the mixture was stirred under solvent-free conditions at room temperature for 6 h. Then CH2Cl2 (10 mL) was added to the reaction mixture. After addition of triphenylphosphine 3 (1 mmol), a mixture of dimethyl acetylenedicarboxylate (DMAD) 4 (1 mmol) in CH2Cl2 (2 mL) was added drop wise. The reaction mixture was then allowed to stir for 24 h. The solvent was removed under reduced pressure, and the residue was washed with diethyl ether (2×3 mL) to afford the pure product. Characteristic analytical and spectroscopic data of dimethyl-2-[4-bromophenyl phenylcarbamate-N-yl]-3-(triphenylphosphoranylidene) butandioate are given below:
Dimethyl-2-[4-bromophenyl phenylcarbamate-N-yl]-3-(triphenylphosphoranylidene) butandioate
Orange Powder, m.p.: 147-148 °C, Yeild 91%, IR(KBr) (νmax cm-1): 1746, 1711, 1653 (C=O), MS m/z, (%): 698 (M+, 15,), Anal Calcd for C37H31BrNO6P, C, 63.80; H, 4.49; N, 2.01, Found: C, 64.02; H, 4.56; N, 2.09.
Major isomer
(54%): 1H NMR (500 MHz, CDCl3): δ 3.13 (3H, s, OCH3), 3.91 (3H, s, OCH3), 5.00 (1H, d, 3JPH = 19.6 Hz, P=C–CH), 6.6-8.1 (24H, m, ArH); 13C NMR (100 MHz, CDCl3): δ 41.07 (d, 1JPC = 135.8 Hz, P=C), 52.37 (s, OCH3), 52.67 (s, OCH3), 61.80 (d,2JPC = 15.0 Hz, P=C–CH), 117.71, 123.25, 126.31 (d, 1JPC = 92.5 Hz, Cipso), 127.70, 127.54, 127.87, 128.60, 128.85 (d, 3JPC = 12.1 Hz, Cmetha), 131.10, 132.17 (d, 4JPC = 2.0 Hz, Cpara), 132.18, 133.59 (d, 2JPC = 10.1 Hz, Cortho), 138.49, 150.71, 153.20 (N–C=O), 168.60 (d, 2JPC = 13.1 Hz, C=O), 173.05 (d, 3JPC = 10.5 Hz, C=O); 31P NMR (162 MHz, CDCl3): δ 24.81.
Minor isomer
(46%): 1H NMR (500 MHz, CDCl3): δ 2.29 (3H, s, OCH3), 3.81 (3H, s, 2OCH3), 5.01 (1H, d, 3JPH = 18.0 Hz, P=C-CH), 6.6-8.1 (24H, m, ArH); 13C NMR (100 MHz, CDCl3): δ 41.08 (d, 1JPC = 134.8 Hz, P=C), 48.98 (s, OCH3), 49.52 (s, OCH3), 62.75 (P=C–CH), 123.25, 126.60, 126.7 (d, 1JPC = 90. 6 Hz, Cipso), 127.54, 127.87, 128.74 (d, 3JPC = 11.1 Hz, Cmetha), 129.95, 131.90, 132.26 (d, 4JPC = 2.0 Hz, Cpara), 132.27, 133.74 (d, 2JPC = 10.1 Hz, Cortho), 138.79, 149.81, 152.68 (N–C=O), 170.08 (d, 2JPC = 17.1 Hz, C=O), 173.54 (C=O); 31P NMR (162 MHz, CDCl3): δ 24.09.
Results and Discussion
Synthesis
Stable phosphorus ylide 5 as a mixture of the two Z-5 major and E-5 minor isomers was generated from the reaction of 4-bromophenol 1, phenylisocyanate 2, triphenylphosphine 3 and DMAD 4 in CH2Cl2 at ambient temperature in excellent yeild (91%). The structures of synthesized products 5 were deduced from their melting point, elemental analysis, IR, 1H, 13C, and 31P NMR and Mass spectra. The mass spectrum of these compounds display molecular ion peak at m/z = 698 (M+). The 1H NMR spectrum of ylide 5 showed the two sharp lines at δ = 3.13 and 3.91 ppm arising from the methoxy groups in the major-diastereoisomer and the two sharp signals at δ = 2.29 and 3.81 ppm in the minor-diastereoisomer. The methine protons of the major and minor rotamers were observed as two doublets at δ = 5.00 (3JPH = 19.6 Hz) and δ = 5.01 (3JPH = 18.0 Hz) ppm, respectively. The aromatic protons were appeared at δ = 6.6–8.1 ppm for major and minor isomers. In 31P NMR spectrum of 5, two signals were observed at δ = 24.81 and 24.09 ppm which were attributed to the major and minor rotamers.
Although the mechanism of this reaction has not been experimentally reported, on the basis of previously reported literatures [7-10,13], a proposed mechanism for the formation of ylide 5 is illustrated in Fig. 2. In this mechanism, reaction between 4-bromophenol 1 and phenylisocyanate 2 gives 4-bromophenyl phenylcarbamate 6. The nucleophilic Michel addition of triphenylphosphine 3 to DMAD 4 generates the zwitterionic 7 and the subsequent protonation of 7 by NH-compound 6 leads to intermediates 8 and 9. Finally, the reaction between the two ionic species 8 and 9 generates stable phosphorus ylide 5 containing of a carbamate derivative.
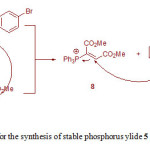 |
Figure 2: The proposed mechanism for the synthesis of stable phosphorus ylide 5 Click here to View figure |
Dynamic effect for the E-5 and Z-5 rotational isomers as a result of restricted rotational process around the carbon–carbon double bond (OMeC=CPPh3)
The rotation around the carbon-carbon double bond in ylide 5 is fairly slow on the NMR time scale at ambient temperature; hence we observed the two E-5 and Z-5 rotational isomers in 1H spectrum. When the temperature was increased considerably higher than 65 ˚C, rotation was too fast. The 1H NMR spectrum of the two E-5 and Z-5 isomers at 50 ˚C showed a broad resonance for the aromatic protons in comparison with the corresponding two doublets at d = 6.7 and 6.8 that was measured at ambient temperature. The aromatic protons coalesce near 62 ˚C (335 K) and are appeared as a broad doublet at 65 ˚C, which is relevant to restricted rotational process around the carbon–carbon double bond (OMeC=CPPh3) as shown in Fig. 3. Moreover, the 1H NMR spectrum of the two E-5 and Z-5 isomers at 42 °C indicated a broad resonance for the methane protons in comparison with two doublets that was measured at ambient temperature. The methine protons coalesce near at 47 °C and are appeared as two sharp lines at 50 °C. Investigation of these phenomena in the phosphorus ylide 5 at variable temperatures allowed us to calculate the rotational energy barrier (∆G≠ = aT[9.972+logTc/Dn]) for the restricted rotational process around the carbon–carbon double bond. The values of ∆G≠ are calculated in different temperatures by using the expression kc= π∆υ/√2 and are accumulated in Table 1. As can be seen in Figs. 4 and 5, these data allow us to draw Arrhenius and Eyring plots. The related activation and kinetic parameters calculated using Figs. 4 and 5 for this process are reported in Table 2.
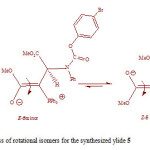 |
Figure 3: Interchangeable process of rotational isomers for the synthesized ylide 5 Click here to View figure |
Table 1: The values of rate constant at various temperature for restricted rotational process around the carbon-carbon double bond
|
Temperature (K) |
kc (s-1) |
DG≠ (kcalmol-1) |
|
293 |
22.04 |
15.33 |
|
298 |
18.56 |
15.70 |
|
318 |
42.73 |
16.28 |
|
323 |
45.86 |
16.50 |
|
328 |
43.62 |
16.79 |
|
333 |
43.40 |
17.06 |
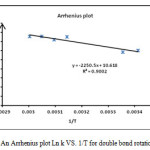 |
Figure 4: An Arrhenius plot Ln k VS. 1/T for double bond rotation C=C Click here to View figure |
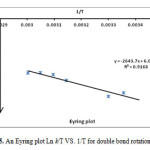 |
Figure 5: An Eyring plot Ln k/T VS. 1/T for double bond rotation C=C Click here to View figure |
 |
Table 2: The key D 1HNMR data and related thermodynamic parameters of activation estimated for restricted rotational process and the related theoretical data around the carbon-carbon double bond C=C Click here to View table |
In order to determine theoretical rotational energy barrier in the rotational interchangeable processes of the two Z– and E-isomers in ylide 5, first their structures were optimized at HF/6-31G(d,p) and B3LYP/6-31G(d,p) levels of theory. This gave structures a and c shown in Fig. 6 for the both E-5 and Z-5, respectively. Then, relative energy versus dihedral angle (O1C2C3P4) is plotted in Fig. 7. This plot was obtained by scanning method at B3LYP/6-31G(d,p) level of theory and each point in Fig. 7 was fully optimized.
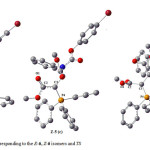 |
Figure 6: Optimized structures corresponding to the E-6, Z-6 isomers and TS Click here to View figure |
As can be seen from Fig. 7, a transition state (TS) is appeared at the top of this plot. Frequency calculations are employed for all states involving transition state and stationary points (b, a and c), respectively. The results are reported in Table 2.
 |
Figure 7: Profile of relative energy in restricted rotational process around the C=C bond for the ylide 5 versus dihedral angel (OCCP) at the B3LYP/6-31G (d,p) level Click here to View figure |
Table 3: Frequency data obtained for TS and GS structures in the restricted rotational process around the C=Cbond
|
Isomer |
First Frequency |
|
E–5 TS Z–5 |
8.51a (8.51)b -69.57(-22.89) 7.29 (9.21) |
aobtained at HF/6-31G(d,p)
bobtained at B3LYP/6-31G(d,p)
First negative frequency value could be considered as a good proof for the confirmation of TS structure. With use of the output data of frequency TS and GS, the values of thermodynamic parameters of activation are calculated and they are accumulated in Table 2. The results indicated that there is a good agreement between the experimental free Gibbs rotational energy barrier (ΔG≠ 12≅”> 15.30 kcal.mol-1) and those obtained at B3LYP/6-31G(d,p) level 16.47 kcal.mol-1 (process E→Z) or 17.21 kcal.mol-1 (process Z®E). It seems that B3LYP/6-31G(d,p) level provide more accurate than HF/6-31G(d,p) level. The results from the B3LYP/6-31G(d,p) level are more compatible with the experimental data. Application of a solvent in the presence of a huge molecule caused so many complications that it became an unsuccessful performance. In total, our attempts failed to employ solvents media. Under this condition (huge molecules with a large number of atoms such as six oxygen, one nitrogen, one bromine and one phosphorus atoms and other atoms along with solvents media), employment of structure optimization was impossible.
Conclusion
The 1H, 13C, and 31P NMR data confirmed synthesis of phosphorous ylide 5 in excellent yield at ambient temperature and indicated two rotational isomers as major Z-5 and minor E-5 forms. The experimental and theoretical data, taken together, indicated that the rotation around the carbon-carbon double bond in the two Z– and E-isomers is too fast at higher temperature (higher 65 °C) on the NMR time scale. When the temperature was considerably reduced down the both Z– and E-isomers are appeared as the two isomers of the synthesized ylide 5. Finally, theoretical calculations exhibited that employment of the DFT method is more preferable than the ab initio method for a huge molecule like the ylide 5 that suffers from crowds of atoms during the interchangeable rotational processes.
Acknowledgements
We gratefully acknowledge the financial support received from the Research Council of the University of Sistan and Baluchestan.
References
- Laszo, P., Organic Reaction: Simplicity and Logic,Wiley. New York, 1995.
- Johnson, A. W., Ylide Chemistry, Academic. London, 1966.
- Hudson, H. R., In the Chemistry of Organophosphorus Compounds: PrimerySecondry and Tertiary Phosphines and Heterocyclic Organophosphorus (III) Compounds, Wiley. New York, 1990.
- Cadogan, J. I. G., Organophosphorus Reagents in Organic Synthesis, Academic Press. New York, 1979.
- Corbridge, D. E. C., Phosphorus: An Outline of Chemistry, Biochemistry and Uses, 15th ed.; Elsevier. Amsterdam, 1995.
- Kolodiazhnyi, O. I. Russ. Chem. Rev. 1994, 66, 225-254.
- Shahraki, M.; Habibi-Khorassani, S. M.; Dehdab, M. RSC Adv. 2015, 5, 52508-52515.
- Yavari, I.; Adib, M.; Jahani-Moghaddam, F.; Bijanzadeh, H.R. Tetrahedron. 2002, 58, 6901–6906.
- Habibi-Khorassani, S.M.; Maghsoodlou, M.T.; Ebrahimi, A.; Zakarianejad, M.; Mohammadzadeh, P.; Shahraki, M. Orient J Chem. 2008, 24(1), 73-82.
- Hallajian, S.; Alipour, E.; Foroughifar, N.; Khalilzadeh, M. A. Orient J Chem. 2014, 30(3), 1311-1315.
- Cherkasov, R. A.; Pudovic, M. A. Russ. Chem. Rev. 1994, 63, 1019-1020.
- Nicolaou, K. C.; Harter, M. W.; Gunzner, J. L.; Nadin, A. Liebigs Ann. 1997, 3, 1283-1301.
- Sreeramulu, J.; Ashokgajapathiraju, P. Orient J Chem. 2014, 30(2), 651-660.
- Engel, R. Synthesis of Carbon-Phosphorus Bond, CRC Press, Boca Raton. FL, 1988.
- Cervinka, O. In Roppoport, Z. Eds., The Chemistry of Enamines, Wiley. New York, 1994.
- Oki, M. In Application of Dynamic NMR Spectroscopy to Organic Chemistry, Eds., VCH. Weinheim, 1985.
- Habibi-Khorassani, S. M.; Ebrahimi, A.; Maghsoodlou M. T.; Asheri, O.; Shahraki, M.; Akbarzadeh, N.; Ghalandarzehi, Y. Int J Chem Kinet. 2013, 45, 596.
- Ali, K. F. Orient J Chem. 2015, 31(1), 239-247.
- Habibi-Khorassani, SM.; Maghsoodlou, MT.; Shahraki, M.; Hashemi-Shahri, Marjan.; Aboonajmi J.; Zarei, T. Iranian Journal of Catalysis. 2014, 4(4), 241-246.
- Habibi-Khorassani, S. M.; Shahraki, M.; Maghsoodlou, M. T.; Erfani, S.; Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015, 145, 410-416.
- Habibi-Khorassani, S.M.; Maghsoodlou, M. T.; Ebrahimi, A.; Mohammadi, M.; Shahraki, M.; Aghdaei, E. J Phys Org Chem. 2012, 25(12), 1328-1335.
- Shahraki, M.; Habibi Khorassani, S. M.; Ebrahimi, A.; Maghsoodlou, M. T.; Paknahad, A., Prog React Kinet and Mech. 2012, 37, 321–343.
- Mofarrah. E.; Habibi-Khorasani, S.M.; Maghsoodlou, M. T.; Shahraki, M. Appl Magn Reson. 2015, 46(10), 1179-1188.
- Shahraki, M.; Habibi-Khorassani, S. M.; Ebrahimi, A.; Maghsoodlou, M.; Ghalandarzehi, Y. Struct Chem. 2012, 24, 623-635.
- Schmidt. M. W.; Baldridge K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen , K. A.; Su, S. J.; Windus, T. L.; Dupuis, M.; Montgomery, J. A. J Comput Chem, 1993, 14, 1347-1363.

This work is licensed under a Creative Commons Attribution 4.0 International License.









