Comparative adsorption of Fe(III) and Cd(II) ions on glutaraldehyde crosslinked chitosan–coated cristobalite
Rahmi*, Fathurrahmi, Irwansyah and Arie Purnaratrie
Chemistry Department, Syiah Kuala University, Banda Aceh. Indonesia
*Corresponding Author Email: rahmi@fmipa.unsyiah.ac.id
DOI : http://dx.doi.org/10.13005/ojc/310427
Article Received on :
Article Accepted on :
Article Published : 06 Dec 2015
In this study, chitosan was crosslinked with glutaraldehyde and coated on the surface of cristobalite through a dip and phase inversion process. The adsorbent was used in batch experiments to evaluate the adsorption of Fe(III) and Cd(II) ions. A maximum adsorption capacity was observed at a glutaraldehyde concentration in sorbent preparation of 1% (w/w). The equilibrium adsorption quantity was determined to be a function of the solution pH, initial concentration and agitation period. Langmuir and Freundlich adsorption models were used to describe adsorption isotherms.
KEYWORDS:chitosan; cristobalite; glurataldehyde; Fe(III) and Cd(II)
Download this article as:| Copy the following to cite this article: Rahmi, Fathurrahmi, Irwansyah, Purnaratrie A. Comparative adsorption of Fe(III) and Cd(II) ions on glutaraldehyde crosslinked chitosan–coated cristobalite. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Rahmi, Fathurrahmi, Irwansyah, Purnaratrie A. Comparative adsorption of Fe(III) and Cd(II) ions on glutaraldehyde crosslinked chitosan–coated cristobalite. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13071 |
Introduction
Removal of heavy metal ions from polluted water is an important issue for health and ecological reasons. Their toxic effect, non-biodegradability and transmission through the food chain justify a strict control of heavy metal discharges into the environment. Many methods have been used to treat such effluents but most of them are either expensive or incapable of removing trace level of heavy metal ions. Adsorption is an effective and economical method for removal of pollutants from wastewater. The adsorption capacity of several low-cost adsorbens has been investigated, mainly biopolymers, which are obtained from renewable sources, present relatively low-cost and selectively adsorbs several metallic ions1-4.
Chitosan is one of the most representative biopolymers, receiving considerable interest for heavy metals removal due to its excellent metal-binding capacities and low cost5-8. Chitosan shows high affinity for metal ions due to the amine (-NH2) and hydroxyl groups (-OH). Chitosan is produced by partially alkaline N-deacetylation of chitin, which can be produced by treating the exoskeleton of shellfish and crustaceans (shrimps, crabs, lobsters, etc.). However chitosan has weak mechanical and chemical properties where it is easily dissolves in most dilute mineral acids. Consequently, its stability needs to be reinforced. Various chitosan-based composites, especially chitosan-natural mineral composites have been used as adsorbents of heavy metal ions. Composite particles as adsorbents are drawing more and more attention due to their specific surface area, chemical and mechanical stability, and a variety of surface and structural properties9-11.
In this study, we used natural mineral namely cristobalite. Cristobalite is a polymorph of quartz, meaning that it is composed of the same chemistry, SiO2, but has a different structure. The possibility of using cristobalite as a material to remove heavy metal ion from water is, however, not commonly explored. Since the polyanionic properties of SiO2 can be expected to contribute to the charged interactions with cationic substances, such as heavy metal ions. We have tested natural cristobalite as an adsorbent for Fe(III) removal, it showed a large adsorption capacity of Fe(III)12.
To enhance the resistance of chitosan against acid, alkali and chemical, chitosan was crosslinked with glutaraldehyde. Crosslinking enhance the resistance of chitosan against acid, alkali and chemical. The crosslinked chitosan also are very stable and maintain their strength even in acidic and basic solutions. These characteristics are very important for an adsorbent so that it can be used in a lower pH environment. Crosslinking also can change the crystalline nature of chitosan and enhance sorption abilities. In this study crosslinked chitosan was coated on the surface of cristobalite. The choice of cristobalite as the substrate is due to its reasonable cost and reasonably interaction with heavy metal ions. The combination of chitosan and cristobalite may work efficiently on removal heavy metal ions from wastewater.
This work concentrates on the study of Fe(III) and Cd(II) ions sorption onto glutaraldehyde crosslinked chitosan-coated cristobalite. The effect of glutaraldehyde concentrations in adsorbent preparation on the performance of Fe(III) and Cd(II) ions uptake was examined. The influence of experimental conditions such as pH and agitation period was also studied. The Langmuir and Freundlich equations were used to fit the equilibrium isotherm.
Materials and Methods
The raw material used was shrimp shells seafood wastes of the city of Banda Aceh, Indonesia. Chitin was extracted from shrimp shells. At first, shrimps shells were washed with water, dried and cut into small pieces. Initial step of chitin extraction was carried out by acetic acid at room temperature for 2 h. This step was followed by filtration, neutralization and washing. Deproteination was performed using alkaline treatment with 2 N sodium hydroxide solution at 60-65oC and then neutralization was carried out by process of washing. Demineralization was followed by 10% hydrochloric acid at room temperature for 3-5 h. A suspension of 1 g chitin in 50 ml aqueous sodium hydroxide, as deacetylation reagent (50% by weight) was mixed at desired temperature (90-95oC). After 3-5 h, the solid was filtered off, washed with water to neutral pH and methanol. It was then dried at 85oC. The chitosan obtained was characterized by using FTIR and the degree of deacetylation was calculated by using baseline method13.
Preparation of glutaraldehyde crosslinked chitosan–coated cristobalite
Immobilization of chitosan on cristobalite was achieved through a dip-coating and phase-inversion method. Chitosan (0.4 g) and glutaraldehyde (with a series of concentration) were dispersed in 100 ml of 2% aqueous acetic acid. The mixture was stirred continously at 60oC for 4 h to obtain the coating solution. The cristobalite (12.5 g) was placed into the chitosan solution at room temperatur for 1 h, and subsequently soaked in a NaOH (2.0 mol/l) solution for 24 h to allow the development and solidification of the glutaraldehyde crosslinked chitosan-coated cristobalite. Finally, The glutaraldehyde crosslinked chitosan-coatd cristobalite were washed extensively with cold and hot distilled water to remove the remaining NaOH, dried at 49oC for 48 h and stored in a desiccator for further use.
Heavy metal adsorption experiments
Bath adsorption experiments were conducted by placing 1.0 g of the glutaraldehyde crosslinked chitosan-coated cristobalite in the flasks containing 20 ml aqueous solution of the known concentration of Fe(III) and Cd(II). These flasks were agitated at a constant shaking rate. The initial pH of the solutions was adjusted with HCl or NaOH to a desired value. The effects of various parameters on the capacity rate of adsorption process were observed by varying pH of the solution, agitation period, and heavy metal ions concentration. The solution volume was kept constant. The amount of metal adsorbed per unit mass (Q) is calculated as
![]()
Where m is the weight of the adsorbent, V is the volume of heavy metal ions solution, Ci (mg/l) and Cf (mg/l) are the concentration of heavy metal ions before and after adsorption experiments, respectively.
Results and Discussion
Characterizations of chitosan and glutaraldehyde crosslinked chitosan-coated cristobalite
Fig. 1 demonstrates the FTIR spectra of chitosan and crosslinked chitosan-coated cristobalite respectively. Absorption band of chitosan at 3444.6 cm-1 is rather intense as consequence of OH and water streching vibrations, whereas the band at 2878.1 cm-1 corresponds to C-H streching vibrations. The bands at 1658.9 and 1378.7 cm-1 may be attributed to deformation vibration of medium intensity of N-H bonds from primary amines and low intensity from C-H bonds of the CH3 group. Based on Fig. 1(a), the deacetylation degree of chitosan was 88,76%. The strong Si-O peak shoulder is at wavenumber 1130.0 cm-1 (Fig. 1(b)).
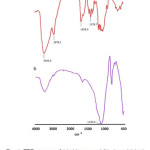 |
Figure 1: FTIR spectra of (a) chitosan and (b) glutaraldehyde crosslinked chitosan-coated cristobalite. Click here to View figure |
The X-ray diffraction pattern of chitosan is shown in Fig. 2(a). It shows two different crystalline structure forms of chitosan. After crosslinking with glutaraldehyde, both of the crystalline structure forms change to be amorph (Fig. 2(b)). The amorphous region will increase the ability of chitosan to adsorp heavy metal ions14. The diffraction of cristobalite tended to cover the reflection of crosslinked chitosan.
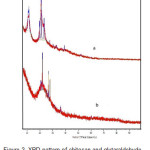 |
Figure 2:XRD pattern of chitosan and glutaraldehyde crosslinked chitosan-coated cristobalite. |
The characterization results confirmed that the formation of new adsorbent glutaraldehyde crosslinked chitosan-cristobalite occured successfully.
Adsorption equilibrium
Glutaraldehyde is one of the most effective crosslinking agents for chitosan. In order to optimize the concentration of glutaraldehyde, the experiments were carried out by using a concentration of glutaraldehyde at 0.0, 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0% (v/v). The glutaraldehyde crosslinked chitosan-coated cristobalite were prepared by two-step process, i.e. crosslinking chitosan with glutaraldehyde followed by coating to cristobalite through a dip and phase inversion process. The resulting adsorbent was examined for adsorption of Fe(III) and Cd(II) ions. The results of this experiment are summarized in Fig. 3.
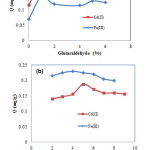 |
Figure 3: Effect of glutaraldehyde in glutaraldehydecrosslinked chitosan-cristobalite preparation (a) and effect of
initial solution pH (b) on the adsorption of Fe(III) and Cd(II). |
It can be seen in Fig. 3(a) that an increase in concentration of glutaraldehyde leads to an increase in the adsorption capacity of Fe(III) and Cd(II). This could be explained by the fact that the crosslinking can enlarge the space between the chitosan chains improving the accessibility of amino groups for metal ions15. The highest adsorption capacity (Q) of both Fe(III) and Cd(II) was obtained when the concentration of glutaraldehyde was 1% (v/v).
The pH of the solutions in the adsorption process is one of the important parameters to be considered because it controls the charge of adsorbent surface as well as the sorbate species in the adsorption solutions. The effect of pH on Fe(III) and Cd(II) adsorption is shown in Fig. 3(b). It shows that the adsorption of Fe(III) and Cd(II) increased with increasing pH and had a maximum adsorption capacity at a pH value of 4.0 for Fe(III) and 5.0 for Cd(II). At low pH environment, more proton will be available to protonate amine groups to form –NH3+, reducing the number of binding sites for the adsorption of Fe(III) and Cd(II) ions. At pH 4 or 5, the lower concentration of H+ ions reduces the protonation, leading the increasing in the adsorption capacity. While, at higher pH, amino groups become deprotonated, repulsion in the polymer chains receded allowing shrinking, reducing the possibility of adsorbate to bind with active sites. Moreover, precipitation of Fe(III) and Cd(II) may occur before adsorption.
Fig. 4 shows the effect of agitation period on the adsorption of Fe(III) and Cd(II) by glutaraldehyde crosslinked chitosan-coated. The adsorption of Fe(III) and Cd(II) increased with agitation period and attained to be constant at about 25 min for Fe(III) and 30 min for Cd(II), implying that equilibrium had been reached.
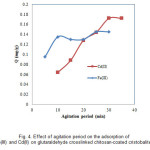 |
Figure 4: Effect of agitation period on the adsorption of Fe(III) and Cd(II) on glutaraldehyde crosslinked chitosan-coated cristobalite. Click here to View figure |
Batch adsorption isotherm experiments were conducted for various initial Fe(III) and Cd(II) concentrations. The adsorption isotherm results were analyzed by the Langmuir model in the linearized form
![]()
Where Q is the amount of adsorbate adsorbed per unit weight of glutaraldehyde crosslinked chitosan-coated cristobalite at equilibrium concentration (mg/g), C is the final concentration in the solution (mg/l), Qmax is the maximum adsorption at monolayer coverage (mg/g), and Ka is the adsorption equilibrium constant (l/mg). The experimental data are plotted as 1/Q versus 1/C and are shown in Fig. 5(a).
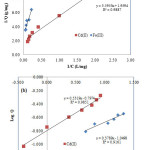 |
Fig. 5. Adsorption isotherms of Fe(III) and Cd(II) on glutaraldehyde crosslinked chitosan-coated cristobalite (a) Langmuir model (b) Freundlich model Click here to View figure |
The Langmuir equation was found to satisfactorily describe the adsorption isotherms of Fe(III) and Cd(II) on glutaraldehyde crosslinked chitosan-coated cristobalite. The numerical values of the Langmuir isotherm constants were determined as Qmax = 0.34 mg/g for Fe(III), Qmax = 0.510 mg/g for Cd(II) and Ka= 0.183 l/mg for Fe(III), Ka= 0.545 l/mg for Cd(II).
Another widely used empirical equation, the Freundlich equation, is based on adsorption on a heterogeneous surface. The equation is represented by:
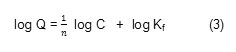
Where Q and C have the same definitions as in equation (2) , Kf is a Freundlich constant representing the adsorption capacity and n is a constant depicting the adsorption intensity. Therefore, Kf and 1/n can be determined from the linear plot of log Q versus log C. Similarly to the Langmuir model, the Freundlich model can also fit the experimental adsorption isotherm data of Fe(III) and Cd(II) very well (Fig. 5(b)). The values of Kf and n can be determined to be Kf = 0.159; n =1.89 and Kf = 0.089; n =2.65 respectively for Fe(III) and Cd(II). If we compare n value of Fe(III) and Cd(II), it shows that the adsorption of Cd(II) more favourable than Fe(III).
It is generally known that the Langmuir model is usually applied to monolayer adsorption processes, and the Freundlich model to multilayer adsorption processes.
Conclusions
In this study, we observed the ability of glutaraldehyde crosslinked chitosan-coated cristobalite for removal Fe(III) and Cd(II) ions aqueous solutions. The amount of adsorption was influenced by glutaraldehyde concentration in the adsorbent preparation, pH of solution, and agitation period. The maximum adsorption amount was observed on glutaraldehyde crosslinked chitosan-coated cristobalite, at pH 4.0-5.0. Equilibrium data agreed very well with Langmuir and Freundlich models.
Acknowledgements
The financial support of the Directorate General of Higher Education, Indonesia (DIKTI), is acknowledged.
References
- Paulino, A.T.; Santos, L. B.; Nozaki, J. (). React. Funct. Polym. 2008, 68, 634-642.
- Barakat, M. A. Arab.J.of Chem. 2011, 4, 361-377.
- Vandenbossche, M.; Jimenez, M.; Casetta, M.; Bellayer,S.; Beaurain, A.; Bourbigot, S.; Traisnel, M. React. Funct. Polym, 2013, 73,53-59.
- Cataldo, S.; Gianguzza, A.; Pettignano, A.; Villaescusa, I. React. Funct. Polym. 2013, 73, 207-217
- Laus, R; Costa, T.G.; Szpoganicz, B.; Favere, V.T. J. Hazard. Mater. 2010, 183, 233-241
- Ngah, W.S.W.; Fatinathan, S. J. Environ. Manage. 2010, 91, 958-969.
- Vieira, R. S.; Beppu, M. M. Adsorp. 2005, 11, 731-736.
- Zhao, F.; Yu, B.; Yue, Z.; Wang, T.; Wen, X.; Liu, Z.; Zhao, C. J. Hazard. Mater. 2007, 147, 67-73.
- Wan, M.W.; Kan, C.C.; Rogel, B.D.; Dalida, M.L.P. Carbohyd. Polym. 2010, 80, 891-899.
- Dalida, M. L. P.; Mariano, A. F. V.; Kan, C.; Tsai, W.; Wan, M. Desalin. 2011, 275, 154-159.
- Futalan, C. M.; Kan, C.; Dalida, M. L.; Hsien, K.; Pascua, C.; Wan, M. Carbohyd. Polym. 2011, 83, 528-536.
- Khairi; Rahmi; Mawaddah, J. Sains Kimia, 2004, 8, 8-11.
- Sabnis, S.; Block, L.H. Polym. Bull. 1997, 39, 67-71.
- Koyama, Y.; Taniguchi, A. J. Appl. Polym. Sci, 1986, 31, 1951-1954.
- Hsien, T. Y.; Rorrer, G. L. Sep. Sci. Technol. 1995, 30, 2455-2475.

This work is licensed under a Creative Commons Attribution 4.0 International License.









