Citrus unshiu flower inhibits LPS-induced iNOS and COX-2 via MAPKs in RAW 264.7 macrophage cells
Min-Jin Kim1, Kyong-Wol Yang2, Eun-Jin Yang1, Sang Suk Kim3, Kyung Jin Park3, Hyun Joo An3, Young Hun Choi3, Nam Ho Lee1, and Chnag-Gu Hyun1*.
1Cosmetic Sciences Center, Department of Chemistry and Cosmetics, Jeju National University, Jeju 690-756, Korea. 2Jeju Love Co., Ltd., 542-5 Haengwon-ri, Gujwa-eup, Jeju 695-975, Korea. 3Citrus Research Station, National Institute of Horticulture and Herbal Science, Seogwipo 697-943, Korea. Corresponding Author Email: cghyun@jejunu.ac.kr
DOI : http://dx.doi.org/10.13005/ojc/310407
Article Received on :
Article Accepted on :
Article Published : 22 Dec 2015
In the present study, we investigated the effects of Citrus unshiu flower on regulatory mechanisms of cytokines and nitric oxide (NO) involved in immunological activity of RAW 264.7 macrophages. Our results indicated that ethyl acetate fraction of Citrus unshiu flower (CUF-EA) downregulated LPS-induced nitric oxide (NO) synthase (iNOS) and cyclooxygenase-2 (COX-2) expression, thereby reducing the production of NO and prostaglandin E2 (PGE2) in LPS-activated RAW 264.7 macrophages. Furthermore, CUF-EA suppressed LPS-induced production of pro-inflammatory cytokines such as interleukin IL-6, and tumor necrosis factor (TNF)-α. To elucidate its anti-inflammatory mechanisms, CUF-EA was investigated as an inhibitor of phosphorylation of mitogen-activated protein (MAP) kinase in LPS-stimulated RAW 264.7 macrophages. As expected, the phosphorylation of MAP kinases (p38, ERK1/2 and JNK) in LPS-stimulated RAW 264.7 macrophages was suppressed by CUF-EA in a dose-dependent manner. These results suggest that the anti-inflammatory properties of CUF-EA might results from inhibition of NO, PGE2, iNOS, COX-2, IL-6 and TNF-α expressions through the down-regulation of phosphorylation of MAPKs in RAW 264.7 macrophages.
KEYWORDS:C. unshiu; lipopolysaccharide; mitogen-activated protein kinases; nitric oxide; prostaglandin E2
Download this article as:| Copy the following to cite this article: Kim M. N, Yang K. W, Yang E. J, Kim S. S, Park K. J, An H. Y, Choi Y. H, Lee N. H, Hyun C. G. Citrus unshiu flower inhibits LPS-induced iNOS and COX-2 via MAPKs in RAW 264.7 macrophage cells. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Kim M. N, Yang K. W, Yang E. J, Kim S. S, Park K. J, An H. Y, Choi Y. H, Lee N. H, Hyun C. G. Citrus unshiu flower inhibits LPS-induced iNOS and COX-2 via MAPKs in RAW 264.7 macrophage cells. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13315 |
Introduction
Citrus unshiu Markovich (Rutaceae) is a seedless and easy-to-peel fruit cultivated primarily on Jeju Island, Korea after introduction from Japan in the 1910s. C. unshiu constitutes more than 30% of the total fruits produced in Korea. The peels, called Jin-pi, have also been used in Traditional Korean Medicine to improve gastroenteric disorders, asthma, and loss of appetite1-2. There are reports that C. unshiu peels and their constituents have diverse pharmacological activities, including anti-anxiety3, recovery of liver function4-5, anti-inflammatory6, anti-allergic7-8, anticancer9, and anti-diabetic10. However, anti-inflammatory effects of the extracts of C. unshiu flowers have not yet been described. Therefore, the anti-inflammatory activities of the extracts of C. unshiu flower were investigated in this study.
Inflammation is the result of the host’s immune response to pathogenic challenges or tissue injuries and functions to restore tissue structure and function to normal states. Inflammation is tightly controlled and self-limiting, with down-regulation of pro-inflammatory proteins and up-regulation of anti-inflammatory proteins11-14. However, if uncontrolled, the inflammatory mediators become involved in the pathogenesis of many inflammatory disorders such as rheumatoid arthritis, diabetes, and cancer 15-16. There have been many attempts to derive new anti-inflammatory agents from natural compounds 17-19.
Inflammatory stimulants such as lipopolysaccharide (LPS) activate production of a variety of inflammatory mediators such as nitric oxide (NO), prostaglandin E2 (PGE2) and pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6. The production of NO and PGE2 by macrophages is a current research interest for the development of new anti-inflammatory agents.
This study was designed to explore the anti-inflammatory effects of C. unshiu flower by measuring its effects on the production of pro-inflammatory factors (TNF-α, IL-6, NO, and PGE2). Furthermore, we sought to elucidate the mechanisms of these anti-inflammatory effects by investigating the role of mitogen-activated protein kinase (MAPK) pathways in RAW 264.7 murine macrophages. To the best of our knowledge, this is the first report of the anti-inflammatory activity of the C. unshiu flower.
Materials and Methods
Plant Material
C. unshiu flowers were collected from the Namwon area (Jeju Island, Korea) in May 2011. The specimen was identified by Dr Young Hun Choi, Citrus Research Station, National Institute of Horticulture and Herbal Science, Jeju, Korea. The specimen was deposited at the Herbarium of the Citrus Research Station.
The specimen materials for extraction were freeze-dried and then ground into a fine powder by using a blender. The dried powder (1.5 kg) was extracted with 80 % ethanol (EtOH; 10 L) at room temperature for 3 days and then evaporated under a vacuum. The evaporated EtOH extract (20 g) was suspended in water (1 L) and fractionated with ethyl acetate (EA; 1 L). The yield and recovery of EA fractions were 2.4 g and 6.2 %, respectively.
Cell Culture
RAW 264.7 murine macrophages were purchased from the Korean Cell Line Bank (Seoul, Korea). These cells were maintained at sub-confluence in a 95% air and 5% CO2 humidified atmosphere at 37°C. The medium used for routine subculture was Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS), penicillin (100 units/mL), and streptomycin (100 μg/mL).
LDH assay for measuring cytotoxicity
RAW 264.7 macrophages (1.8 × 105 cells/mL) were plated in 24-well plates and incubated for 18 h prior to treatment with the indicated concentrations of extract samples for 2 h. Macrophages were then challenged with LPS (1 μg/mL) for an additional 18 h. The release of lactate dehydrogenase (LDH) from the macrophages was used to assess cytotoxicity by using an LDH cytotoxicity detection kit (Promega, Madison, WI, USA). This method determines LDH activity from the production of NADH during the conversion of lactate to pyruvate. The optical density of the solution was measured at a wavelength of 490 nm by using an ELISA plate reader (Bio-TEK Instruments Inc., Vermont, WI, USA). The cytotoxicity percentage was determined relative to the control group. All experiments were performed in triplicate.
Nitric Oxide Determination
Nitrite concentration in the culture medium was measured as an indicator of nitric oxide production according to the Griess reaction method, as previously described19. In brief, RAW 264.7 macrophages (1.8 × 105 cells/mL) were plated in 24-well plates and incubated for 24 h. Macrophages were pre-treated for 2 h with 10 μΜ 2-amino-4-methylpyridine (positive control), 20 μΜ dexamethasone (negative control), or CFU extracts in water, EtOH, or EA or a water fraction at concentrations of 25, 50 and 100 μg/mL), Macrophages were then challenged with LPS (1 μg/mL) for an additional 18 h. Equal volumes of culture medium and Griess reagent (1 % sulfanilamide in 5 % phosphoric acid and 0.1 % naphtylethylenediamine dihydrochloride in distilled water) were mixed. The absorbance at 540 nm was determined with an ELISA plate reader (Bio-TEK Instruments Inc., Vermont, WI, USA). The absorption coefficient was calibrated using a sodium nitrite solution standard.
Measurement of PGE2 Production
RAW 264.7 macrophages were plated in a 24-well cell culture plate at a density of 1.8 × 105 cells/well and incubated for 18 h. Macrophages were then stimulated with and LPS (1 μg/mL) in the presence of CFU-EA (25, 50 and 100 μg/mL) or in the absence of CFU-EA (control) for 24 h. The culture medium was collected and assayed with ELISA kit (R&D Systems, Minneapolis, MN, USA). Culture medium was incubated in a goat anti-mouse IgG coated plate with acetylcholinesterase linked to PGE2 and PGE2 monoclonal antibody for 18 h at 4 °C. The plate was emptied and rinsed five times with wash buffer contained in the kit. Two hundred milliliters of substrate reagent was added to each well and incubated for 1 h at 37 °C. The plate was read at 405 nm on an ELISA plate reader (Bio-TEK Instruments Inc., Vermont, WI, USA). The PGE2 concentration of each sample was determined according to the standard curve.
Measurement of Pro-inflammatory Mediators
The secretion of TNF-α and IL-6 in macrophage cultures was measured using a commercial mouse TNF-α or IL-6 ELISA kit (R&D Systems Inc., Minneapolis, MI, USA). Macrophages (1.8 × 105 cells/mL) were cultured in 24-well plates in the presence or absence of CFU-EA (25, 50 and 100 μg/mL).and LPS (1 μg/mL), and then incubated for 24 h. Supernatant samples were obtained 16 h later and frozen until subjected to ELISA analysis following the manufacturer’s protocol. The TNF-α and IL-6 production in colon homogenates was quantified by reading the absorbance at 540 nm on an ELISA plate reader (Bio-TEK Instruments Inc., Vermont, WI, USA).
Western blot Analysis
RAW 264.7 macrophages were pre-incubated for 18 h in a 6-well cell culture plate at a density of 1.0 × 106 cells/well before being stimulated with LPS (1 μg/mL) in the absence or presence of CFU-EA (25, 50 and 100 μg/mL) extracts for 24 h. After incubation, the macrophages were collected and washed twice with cold phosphate-buffered saline (PBS). Macrophages were lysed in a lysis buffer (1x RIPA [Upstate USA Inc., NY, USA], 1 mM Na3VO4, 1 mM NaF, 1 mM phenylmethylsulphonyl fluoride, 1 μg/mL aprotinin, 1 μg/mL pepstatin, and 1 μg/mL leupeptin) and kept on ice for 1 h. The cell lysates were centrifuged at 15,000 rpm and 4 °C for 15 min, and the supernatants were stored at -70 °C until use. Protein concentrations were then determined using a Bradford Assay (Bio-Rad, Richmond, CA, USA). Aliquots of the lysates (30~50 μg of protein) were separated on an 8~12 % SDS polyacrylamide gel. After electrophoresis, the proteins were electrotransferred to polyvinylidene fluoride (PVDF) membranes (BIO-RAD, HC, USA), blocked with 5 % non-fat milk in TBS-T buffer, and blotted with each primary antibody (1:1,000, except for iNOS (1:5,000) and β-actin (1:10,000)) and the corresponding secondary antibody (1:5,000 or 1:10,000) according to the manufacturer’s instructions. Immunoreactive proteins were detected using an enhanced chemiluminescence (ECL) Western blotting detection kit (Amersham Biosciences, NJ, USA).
Results
Effect of CUF-EA on cell viability and inhibition of NO production
We initially examined the inhibitory effects of the extracts of C. unshiu flower (CUF) on the production of the inflammatory mediator, NO. The amount of produced NO was indicated by amount of nitrite (a stable metabolite of NO) that accumulated in LPS-exposed cells in the presence of each type of CUF. The ethyl acetate fraction of C. unshiu flower extract (CUF-EA) strongly inhibited LPS-induced NO production (Fig. 1). In contrast, the 80% EtOH and water extracts of C. unshiu did not inhibit LPS-induced NO production. In addition, the cytotoxicity of extracts of C. unshiu were evaluated by a lactate dehydrogenase (LDH) assay after the cells were incubated for 24 h in the presence of extracts of C. unshiu and LPS. As shown in Figure 1, there was no cytotoxicity. Thus, the inhibitory effect of all CUF-EA concentrations on NO production could not be attributed to cytotoxic effects. These results suggest that the CUF-EA exerted anti-inflammatory effects.
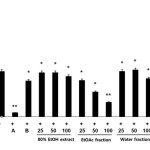 |
Figure 1: Effects of Citrus unshiu flower (CUF) on cytotoxicity and nitric oxide (NO) production in LPS-stimulated RAW 264.7 macrophages NO production was assayed in the culture medium of macrophages (1.8 x 105 cell/mL) stimulated with LPS (1 μg/ml) for 24 h in the presence of CFU extracts in water, EtOH, or EA or a CFU water fraction at concentrations of 25, 50 and 100 μg/mL. The positive control (A) was 10 μΜ 2-amino-4-methylpyridine and the negative control (B) was 20 μΜ dexamethasone. Data were expressed as means ± S.D. of three determinations. *, P<0.05; **, P<0.01 compared to positive control. Click here to View figure
|
Effect of CUF-EA on LPS-induced PGE2 production
To evaluate the effects of CUF-EA on LPS-induced PGE2 production in RAW 264.7 macrophages, the production of PGE2 was measured using ELISA. Stimulation of cells with LPS (1 μg/mL) resulted in a significant increase in PGE2 production compared with un-stimulated control cells. Furthermore, pretreatment with CUF-EA (10, 50, and 100 μg/mL) markedly inhibited LPS-induced PGE2 production (Figure 2).
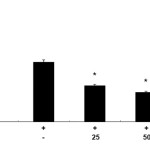 |
Figure 2: Effects of the ethyl acetate fraction of Citrus unshiu flower (CUF-EA) on LPS-stimulated PGE2 production Macrophages (1.8 x 105 cell/mL) were pre-incubated for 18 h and then stimulated with LPS (1 μg/ml) for 24 h in the presence or absence of CFU-EA (25, 50 and 100 μg/mL). PGE2 production was analyzed with an ELISA assay. The data were expressed as means ± S.D. of three determinations. *,P<0.05; **, P<0.01 compared to the positive control (LPS alone). |
Inhibitory Activities of CUF-EA on LPS-Activated iNOS and COX-2 Expression
To understand whether CUF-EA can inhibit LPS-induced activation of iNOS and COX-2 protein, western blotting was performed. As shown in this experiment, the iNOS protein was strongly induced by LPS and CUF-EA showed a decrease in iNOS protein expression in a concentration-dependent manner. In order to clarify whether the inhibitory effect of CUF-EA on PGE2 release could result from a decreased COX-2 protein level, we further examined the effect of CUF-EA on LPS-induced COX-2 protein levels by Western blot analysis. As shown in this experiment, the COX-2 protein was strongly induced by LPS. CUF-EA slightly suppressed the induction of COX-2 (Figure 3).
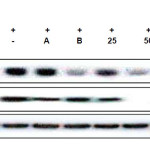 |
Figure 3: Effects of CUF-EA on LPS-induced iNOS and COX-2 protein levels in RAW 264.7 macrophages Macrophages (1.8 x 105 cell/mL) were pre-incubated for 18 h, and then cells were stimulated with LPS (1 μg/mL) for 24 h in the presence of CFU-EA (25, 50 and 100 μg/mL). The positive control (A) was 10 μΜ 2-amino-4-methylpyridine and the negative control (B) was 20 μΜ dexamethasone. iNOS and COX-2 protein levels were determined by immunoblotting with actin as a positive control. Click here to View figure |
Effect of CUF-EA on LPS-induced TNF-α and IL-6 production
In this study, we used ELISA kits to measure the level of TNF-α (Figure 4A) and IL-6 (Figure 4B) in the culture supernatants. Compared with the control group, treatment of RAW 264.7 cells with LPS alone led to a significant increase in cytokine production. However, compared with the LPS group, the levels of TNF-α and IL-6 in CUF-EA-pretreated, LPS-stimulated cells were reduced significantly in a dose dependent manner.
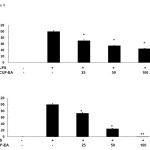 |
Figure 4: Effects of CUF-EA on production of pro-inflammatory cytokines in LPS-induced RAW 264.7 macrophages Macrophages (1.8 x 105 cell/mL) were pre-incubated for 18 h, and then stimulated with LPS (1 μg/mL) for 24 h in the presence or absence of CFU-EA (25, 50 and 100 μg/mL). TNF-α and IL-6 production were analyzed by ELISA. The data are expressed as means ± S.D. of three determinations. *, P<0.05; **, P<0.01 compared to positive control (LPS alone). |
Suppression of MAPK phosphorylation by CUF-EA in LPS-stimulated RAW 264.7 macrophages
Because CUF-EA most markedly inhibited the secretion of inflammatory mediators, we focused on the mechanism by which CUF-EA inhibits LPS-induced inflammation in macrophages. MAPKs are known as the major signaling pathways that regulate the induction of TNF-α and IL-620. Therefore, the phosphorylation of MAPKs c-Jun N-terminal kinase (JNK), extracellular signal regulated kinase (ERK), and p38, were examined in RAW 264.7 macrophages treated with LPS. As shown in figure 5, LPS-induced phosphorylation of JNK, ERK, and p38 were all inhibited by CUF-EA treatment in a dose-dependent manner.
 |
Figure 5: Effects of CUF-EA on the protein levels of p-38, JNK, and ERK in LPS-induced RAW 264.7 cells Macrophages (1.0 x 106 cell/mL) were pre-incubated for 18 h and then stimulated with LPS (1 μg/mL) for 24 h in the presence or absence of CFU-EA (25, 50 and 100 μg/mL). p-JNK, JNK, p-ERK, ERK, pp-38, and p-38 were determined by immunoblotting with β-actin as a positive control. Click here to View figure |
Discussion
C. unshiu is a kind of citrus fruit in the Rutaceae family. It is composed of rind and sarcocarp and includes various bioactive substances such as essential oils, carotenoids, cellulose, pectin, limonoid. In recent studies, it has been reported that flavonoids are also contained in C. unshiu peel extracts that have anti-oxidant, anti-cancer, and anti-inflammatory activity. In addition, the flavonoid content changes during maturation of C. unshiu and is high in premature C. unshiu. However, anti-inflammatory effects of the extracts of C. unshiu flowers have not yet been described.
The present study was designed to elucidate the pharmacological and biological effects of the flower of C. unshiu on the production of pro-inflammatory cytokines and inflammatory mediators in macrophages.
Inflammation is a host response to injury related to chemical or microbiological toxins. LPS is expressed in the outer membrane of gram-negative bacteria, and plays a key role in the initiation of inflammation by producing inflammatory mediators including NO, TNF-α, and IL-621. Our results indicated that each type of C. unshiu extract we used in this study reduced the production of inflammatory mediators stimulated by LPS. Especially, the ethyl acetate fraction of C. unshiu flower (CUF-EA) showed the most significant suppression of NO production. These results suggest that CUF-EA may have a most potent therapeutic effect on the LPS-induced inflammation.
Focusing on the CUF-EA, we examined its anti-inflammatory mechanism in LPS-stimulated RAW 264.7. Similar to NO, an excessive production of PGE2 is correlated with many inflammatory disorders22. PGE2 produced by COX-2 is known to play a key role in inflammatory processes including pain, fever, swelling, and tenderness. Therefore, newly developed selective COX-2 inhibitors share a common therapeutic action on acute and chronic inflammation through suppression of PGE2 production and have been used as useful anti-inflammation drugs23. Our present study demonstrates that CUF-EA decreases the production of PGE2 as well as COX-2 expression in LPS-stimulated RAW264.7 cells, and CUF-EA dose dependently down-regulated iNOS expression. Among the pro-inflammatory cytokines, TNF-α is regarded as one of the most important mediators for producing an inflammatory response that can elicit acute inflammatory diseases and cause tissue destruction24. IL-1β and IL-6 also elicit inflammatory responses, leading to inflammation25. In the present study demonstrated that CUF-EA strongly inhibited LPS-induced TNF-α, IL-6 production.
LPS activation of mammalian cells occurs through its binding to TLR-4 and activation of its downstream signaling pathway, including MAPK pathways26. MAPKs are protein Ser/Thr kinases, which are also known to play major roles in the transmission of inflammatory signals to the nucleus27. LPS stimulation induces the phosphorylation and activation of three types of MAPKs: JNK, ERK, and p38 MAPK. JNK signaling regulates the expression of iNOS28, whereas ERK signaling and p38 signaling upregulate iNOS expression and the production of proinflammatory cytokines including TNF-α and IL-6 in LPS-stimulated macrophages29. Our data showed that CUF-EA dose-dependently inhibited the phosphorylation of three types of MAPKs.
In conclusion, the results of the present study provide the first evidence that CUF-EA inhibit LPS-induced NO and PGE2 production in macrophages. These inhibitory effects of CUF-EA were found to be associated with pro-inflammatory cytokines (TNF-α, and IL-6) via inhibition of the phosphorylation of MAPKs, suggesting a possible approach for the treatment of inflammatory diseases. In future studies, we will investigate the ability of CUF-EA to stimulate the immune system in an in vivo model system.
Conflict of interest
There is no conflict of interest.
Financial support
This research was supported by a grant from the Ministry of Trade, Industry and Energy, Republic of Korea through the Korea Industrial Complex Corp. (2011).
References
- Lyu, J.H.; Lee, H.T.; Archives of Pharmacal Research, 2013, 36, 641-648.
- Park, H.J.; Jung, U.J.; Cho, S.J.; Jung, H.K.; Shim, S.; Choi, M.S.; Journal of Nutritional Biochemistry, 2013, 419-427.
- Ito, A.; Shin, N.; Tsuchida, T.; Okubo, T.; Norimoto, H.; Molecules, 2013, 18, 10014-10023.
- Park, H.Y.; Choi, H.D.; Eom, H.; Choi, I.; Food Chemistry, 2013, 139, 231-240.
- Park, H.Y.; Ha, S.K.; Eom, H.; Choi, I.; Food and Chemical Toxicology, 2013, 55, 637-644.
- Oh, Y.C.; Cho, W.K.; Jeong, Y.H.; Im, G.Y.; Yang, M.C.; Hwang, Y.H.; Ma, J.Y.; American Journal of Chinese Medicine, 2012, 40, 611-629.
- Takayanagi, K.; Frontiers in Neurology, 2011, 2, 67.
- Takayanagi, K.; Morimoto, S.; Shirakura, Y.; Mukai, K.; Sugiyama, T.; Tokuji, Y.; Ohnishi, M.; Journal of Agricultural and Food Chemistry, 2011, 59, 12342-12351.
- Lee, S.; Ra, J.; Song, J.Y.; Gwak, C.; Kwon, H.J.; Yim, S.V.; Hong, S.P.; Kim, J.; Lee, K.H.; Cho, J.J.; Park, YS.; Park, C.S.; Ahn, H.J.; Journal of Ethnopharmacology, 2011,133, 973-979.
- Fujita, T.; Shiura, T.; Masuda, M.; Tokunaga, M.; Kawase, A.; Iwaki, M.; Gato, T.; Fumuro, M.; Sasaki, K.; Utsunomiya, N.; Matsuda, H.; Journal of Natural Medicines, 2008, 62, 202-206.
- Park, H.H.; Kim, M.J.; Li, Y.; Park, Y.N.; Lee, J.; Lee, Y.J.; Kim, S.G.; Park, H.J.; Son, J.K.; Chang, H.W.; Lee, E.; International Immunopharmacology, 2013, 15, 296-302.
- Lee, J.; Yang, G.; Lee, K.; Lee, M.H.; Eom, J.W.; Ham, I.; Choi, H.Y.; BMC Complementary and Alternative Medicine, 2013, 13, 92.
- Jin, H.; Zhu, Z.G.; Yu, P.J.; Wang, G.F.; Zhang, J.Y.; Li, J.R.; Ai, R.T.; Li, Z.H.; Tian, Y.X.; Zhang, W.X.; Wu, S.G.; Phytotherapy Research, 2012, 26, 1320-1326.
- Yang, E.J.; Ham, Y.M.; Yang, K.W.; Lee, N.H.; Hyun, C.G.; Scientific World Journal, 2013, 2013,712303.
- Kim, S.H.; Lee, T.H.; Lee, S.M.; Park, J.H.; Park, K.H.; Jung, M.; Jung, H.; Mohamed, M.A.; Baek, N.I.; Chung, I.S.; Kim, J.; Experimental Biology and Medicine (Maywood), 2015, 240, 946-954.
- Kim, K.N.; Kang, M.C.; Kang, N.; Kim, S.Y.; Hyun, C.G.; Roh, S.W.; Ko, E.Y.; Cho, K.; Jung, W.K.; Ahn, G.; Jeon, Y.J.; Kim, D.; Environmental Toxicology and Pharmacology, 2015, 39, 962-968.
- Chen, T.Y.; Sun, H.L.; Yao, H.T.; Lii, C.K.; Chen, H.W.; Chen, P.Y.; Li, C.C.; Liu, K.L.; Food and Chemical Toxicology, 2013, 55, 257-264
- Yang, G.; Ham, I.; Choi, H.Y.; Food and Chemical Toxicology, 2013, 58, 124-132.
- Kim, M.J.; Kim, S.J.; Kim, S.S.; Lee, N.H.; Hyun, C.G.; EXCLI Journal, 2014, 13, 123-136.
- Ahn, K.S.; Noh, E.J.; Zhao, H.L.; Jung, S.H.; Kang, S.S.; Kim, Y.S.; Life Sciences, 2005, 76, 2315-2328.
- Guha, M.; Mackman, N.; Cell Signaling, 2001, 13, 85-94.
- Rampton, D.S.; Sladen, G.E..; Youlten, L.J.; Gut, 1980, 21, 591-596.
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N.; International Journal of Biochemistry and Cell Biology, 2006, 38, 1654-1661.
- Choi, J., Callaway, Z., Kim, H. B., Fujisawa, T., Kim, C. K.; Pediatric Allergy and Immunology, 2010, 21, 474-479.
- Eliav, E., Benoliel, R., Herzberg, U., Kalladka, M., Tal, M.; Brain, Behavior, and Immunity, 2009, 23, 474-484
- Kawai, T.; Akira, S.; Cell Death and Differentiation, 2006, 13, 816-825.
- Guha, M.; Mackman, N.; Cell Signaling, 2001, 13, 85-94.
- Uto, T.; FujiiM, Hou D.X. Biochemical Pharmacology, 2005, 70, 1211-1221.
- Ajizian, S.J.; English, B.K.; Meals, E.A.; Journal of Infectious Diseases, 1999, 179, 939-944.

This work is licensed under a Creative Commons Attribution 4.0 International License.









