Chemical thermodynamics applied to the synthesis of tropinone
Sandra Cotes*1 and José Cotuá2
1Universidad del Norte, Km 5 Antigua Vía a Puerto Colombia, Barranquilla, Colombia.
2Universidad del Atlántico, Km 7 Antigua Vía a Puerto Colombia, Barranquilla, Colombia.
Corresponding Autor Email: scotes@uninorte.edu.co
DOI : http://dx.doi.org/10.13005/ojc/310410
Article Received on :
Article Accepted on :
Article Published : 25 Nov 2015
The potential energy surface in Robinson’s tropinone synthesis was calculated using molecular modeling tools. The reaction mechanism was studied, and the stability of the accepted reactive species was evaluated. The findings indicated the amino alcohol intermediate had greater stability than the other proposed intermediates. It was also found that in the presence of dicarboxylic acetone, the activation energy decreased by 69.30 kcal/mol compared to that of the reaction in the presence of acetone.
KEYWORDS:Robinson´s tropinone synthesis; reaction mechanism; potential energy surface
Download this article as:| Copy the following to cite this article: Cotes S, Cotuá J. Chemical thermodynamics applied to the synthesis of tropinone. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Cotes S, Cotuá J. Chemical thermodynamics applied to the synthesis of tropinone. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12722 |
Introduction
There is an increasing interest in the synthesis and biosynthesis of alkaloids derived from tropane because of the biological importance of many of these compounds. Many synthetic studies have focused on cocaine analogues derived from tropinone1. The tropinone synthesis reported by Robinson2 is highlighted by the simplicity of the used reagents and its biomimetic. Robinson’s tropinone synthesis has become a classic organic synthesis, in which a simple condensation reaction between succinaldehyde, methylamine, and acetone takes place (see Figure 1)3.
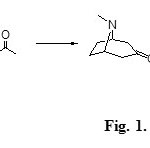 |
Figure 1: Robinson’s synthesis Click here to View figure |
The typical experimental procedure involves two steps: the aldehyde and the amine react to form an imine intermediate, which in turn reacts with the substrate containing an acidic hydrogen in the second step. This stepwise procedure shows benefits in terms of better yields and improved reaction rates compared with the conventional Mannich reactions4 and also allows for a better analysis of the reaction mechanism (see Figure 2).
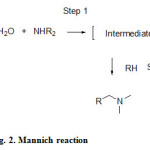 |
Figure 2: Mannich reaction Click here to View figure |
The reported reaction yield by Robinson was low because of the low acidity of the acetone. The use of calcium dicarboxylic acetone or ethyl dicarboxylic acetone in place of acetone under acidic conditions improved the yield to up to 40%. Schöpf et al.5 obtained 70–85% yields by conducting the reaction at pH 7 with dicarboxylic acetone.
The reaction mechanism proposed for Robinson’s synthesis is similar to that of an aldol condensation (Figure 3). Two consecutive Mannich reactions take place: one intermolecular and the other intramolecular. Three types of intermediates (imine, iminium cation, and amino alcohol) have been proposed for Mannich reactions. The first step involves the nucleophilic addition of methylamine to the aldehyde and loss of water to generate the imine intermediate (I). Intramolecular addition of I to an aldehyde closes the ring, producing the iminium cation (II). An alternative intermediate is an amino alcohol (III)6, which in acidic conditions produces II. Then, the intermolecular Mannich reaction between II and the enolate of dicarboxylic acetone (IV) takes place. Before ring closing could occur, enolate and imine form by a new pathway that involves water loss. Finally, the loss of the two carboxylic groups occurs to afford tropinone. Many works have been performed on the mechanism of this reaction catalyzed by metals or imines7. Mondal et al.8 carried out a theoretical study of the Robinson’s reaction mechanism in the absence of chiral media. In their calculations based on HF/6-31G*, the intermolecular Mannich reaction between II and the enolate of dicarboxylic acetone (IV) was studied, different transition states of the mechanism at this stage were found (Figure 3).
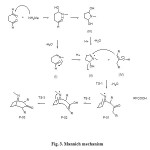 |
Figure 3: Mannich mechanism |
Thermodynamic and molecular properties such as heats of formation (ΔHf), entropies (S) and Gibbs energies of formation (ΔGf) are invaluable parameters to study reactions mechanisms but theoretical calculations of these values usually demands the use of highly parameterized quantum methods. Pankratov9 has demonstrated that semiempirical methods reproduce correctly the trends of thermodynamic and molecular properties in series of organic species including bridgehead bicyclo-octanes.
In this paper, we introduce a semi-empirical study of the complete potential energy surface for the synthesis of tropinone in the gas phase. This is for the first time that the stability of the three most common intermediates (I, II, and III) are studied.
Computational Details
The potential energy surface of the synthesis of tropinone was calculated using the PM3 semi-empirical method. The minimum energy conformer and transition states in the gas phase were characterized by frequency analysis.
The equations used for the calculation of the equilibrium constant Kp are as follows:
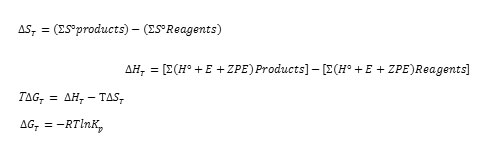
Results and Discussion
The energy profile section determined by Mondal et al.9 is in full agreement with the findings that we obtained with the semi-empirical method PM3 (Figure 4).
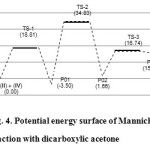 |
Figure 4: Potential energy surface of Mannich reaction with dicarboxylic acetone Click here to View figure |
The activation energies for the three transition states TS-1, TS-2, and TS-3 are 18.81, 38.33, and 15.08 kcal/mol, respectively, and the energy of the global reaction is 15.43 kcal/mol. We have used a single water molecule as catalyst in TS-2; however more than one molecule may be involved in the tautomerisation.
C-C bond formation usually takes place through transition states with eclipsed conformations; each transition state can exist in its enantiomeric form being of the same energy in an achiral environment. The presence of an asymmetric and prochiral centre in the iminium cation (II) produces two diastereotopic faces for the attack of the dicarboxylic acetone, which also possesses two enantiotopic faces. Therefore, four diastereomeric transition states will be generated for each R and S configurations of the iminium cation. However, as our goal is not to study the stereoselectivity, we work with only one isomeric conformation, i.e., the transition states were generated based on the configuration of the S iminium cation and nucleophilic attack of the si face of the enolate to the re face of the iminium cation. The energy profile for the overall process is shown in Figure 4, and the optimized structures of the transition states are shown in Figure 5.
Table 1: Equilibrium constants and total energies of intermediates (I, II, and III)
| Step 1 | Kp | E (kcal/mol) |
| Imine (I) | 0.07 | -82.66 |
| Iminium cation (II) | 4.11×1093 | 64.97 |
| Amino alcohol (III) | 5.06×1010 | -102.15 |
 |
Scheme 1 Click here to View scheme |
According to our results, among the three proposed intermediates for the first Mannich reaction—an imine (I), an iminium cation (II), and the amino alcohol (III)—the most stable is the amino alcohol intermediate (III) (see Table 1).
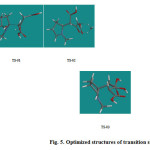 |
Figure 5: Optimized structures of transition states |
Our thermodynamic study in the gas phase reveals that the formation of the more stable intermediate, promotes the loss of a water molecule in acidic media to generate the iminium cation without the formation of (I) (see Kp, Table 1). The second stage of tropinone synthesis starts from the iminium cation which is a spontaneous process with a favourable global equilibrium constant of 5.72×1019.
In our work, the transition state conformations remained largely unaffected by the presence of the carboxylic groups which is similar to the result reported by Mondal et al.8 However, in the absence of the carboxylic groups, the reaction proceeds with activation energies of 88.11 kcal/mol for TS-1, 44.88 kcal/mol for TS-2, and 10.06 kcal/mol for TS-3. The energy of the overall reaction is 91.44 kcal/mol (Figure 6). Thus, it is clear that the carboxylic acetone favours the overall reaction by 69.30 kcal/mol for TS-1. The greater total activation energy for TS-2 when acetone is used is also notable (Figure 4 and 6).
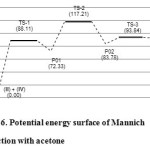 |
Figure 6: Potential energy surface of Mannich reaction with acetone Click here to View figure |
Conclusions
The Mannich reaction in the synthesis of tropinone and its potential energy surface has been evaluated using the PM3 semi-empirical calculation method with satisfactory results. The rate-determining step in the reaction is the enolisation of intermediate P-01 through transition state TS-2. Increasing the acidity of the acetone reduces considerably the activation energy of the rate determining step. Among the most commonly proposed intermediates (I, II, and III) for the Mannich reaction it was found that the formation of the amino alcohol intermediate (III) is favoured over the imine intermediate (I).
Acknowledgments
This work was supported by the Dirección de Investigación, Desarrollo e Innovación (DIDI) of the Universidad del Norte.
References
- a) Qiao, H.; Zhu, L.; Lieberman, B. P.; Zha, Z.; Plössl, K.; Kung H. F. Bioorganic & Medicinal Chemistry Letters. 2012, 13, 1, 4303-4306 b) Holloway, G.A.; Parisot, J.P.; Novello, P.M.; Watson, K.G.; Armstrong, T.; Thompson, R.C.A.; Street, I.P.; Baell, J. B. Bioorganic & Medicinal Chemistry Letters. 2010, 20, 6, 1816-1818 c) Pin, F.; Vercouillie, J.; Ouach, A.; Mavel, S.; Gulhan, Z.; Chicheri, G.; Jarry, C.; Massip, S.; Deloye, J-B.; Guilloteau, D.; Suzenet, F.; Chalon, S.; Routier, S. European Journal of Medicinal Chemistry. 2014, 82, 214-224 d) Słowiński, T.; Stefanowicz, J.; Dawidowski, M.; Kleps, J.; Czuczwar, S.; Andres-Mach, M.; Łuszczki, J. J.; Nowak, G.; Stachowicz, K.; Szewczyk, B.; Sławińska, A.; Mazurek, A. P.; Mazurek, A.; Pluciński, F.; Wolska, I.; Herold F. European Journal of Medicinal Chemistry. 2011, 46, 9, 4474-4488 e) Mavel, S.; Mincheva, Z.; Méheux, N.; Carcenac, Y.; Guilloteau, D.; Abarbri, M.; Emond P. Bioorganic & Medicinal Chemistry. 2012, 20, 4, 1388-1395
- Robinson, R. A synthesis of tropinone, J. Chem. Soc. 1917, 111, 762-768
- a) March, J.; Smith. March´s advanced organic chemistry, Sixth edition; John Wiley. New Jersey, (2007). b) M. B. Tramontini M.; Angiolini, L. In Mannich Bases: Chemistry and Uses; CRC Press, Boca Raton, Florida, 1994 261
- Holy, N.; Fowler, R.; Burnett, E. Lorenz, R. Tetrahedron. 1979, 33, 5, 613-619
- Schöpf, C.; Lehmann, G. Annalen. 1935, 518, 1-36
- a) Henderson, K. W.; Dorigo, A. E.; Liu, Q.-Y.; Williard, P. G.; Schleyer, P. V. R.; Bernstein, P. R. J. Am. Chem. Soc. 1996, 118, 6, 1339-1347; b) Li, Y.; Paddon-Row, M. N.; Houk, K.N. J. Org. Chem. 1990, 55, 2, 481-493 c) Bernardi,A.; Capelli, A.M.; Gennari, C. J. Org. Chem. 1990, 55, 11, 3576-3581
- a) Hoang, L.; Bahmanyar, S.; Houk, K. N.; List, B. J. Am. Chem. Soc. 2003, 125, 16-17 b) Bahmanyar, S.; Houk, K. N. J. Am. Chem. Soc. 2001, 123, 51, 12911-12912 c) Q. Guo, J. C.-G. Zhao, Org. Lett. 2013, 15, 508-511 d) Kobayashi, S.; Kiyohara, H.; Yamaguchi, M. J. Am. Chem. Soc. 2011, 133, 708-711 e) Hatano, M.; Horibe, T.; Ishihara, K. J. Am. Chem. Soc., 2010, 132, 56-57 f) Bandar, J. S.; Lambert, T. H. J. Am. Chem. Soc., 2013, 135, 11799-11802 g) Salter, M. M.; Kobayashi, J.; Shimizu, Y.; Kobayashi, S. Org. Lett. 2006, 8, 3533-3536
- Mondal, N.; Mandal, S.; Das, G.; Mukherjee, S. J. Chem. Research (S). 2003, 580-583
- Pankratov, A. N. Theochem. 1998, 453, 7-15

This work is licensed under a Creative Commons Attribution 4.0 International License.









