Use of Variamine Blue dye in Spectrophotometric determination of Water Soluble Cr(VI) in Portland Cement
Devesh K. Sharma* and Rekha Sharma
School of Physical Science, Lovely Professional University, Phagwara, India. Corresponding Author Email: devsarita@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310448
Article Received on :
Article Accepted on :
Article Published : 04 Dec 2015
Variamine blue dye as chromogenic reagent was used for Portland cement samples in determination of soluble hexavalent chromium. This method was based on the reaction of Cr(VI) with potassium iodide in acidic medium to liberate iodine, which oxidized variamine blue to form a violet colored species having an absorption maximum 556 nm. The extraction of soluble Cr(VI) for quantification in cement was done according to European method. The validity of this method was thoroughly examined by comparing with standard DPC method as well as the accuracy of the method was checked using a standard reference material of National Institute of Standards & Technology (NIST), USA.
KEYWORDS:Soluble Cr(VI); Portland cement; Variamine blue; Spectrophotometer
Download this article as:| Copy the following to cite this article: Sharma D. K, Sharma R. Use of Variamine Blue dye in Spectrophotometric determination of Water Soluble Cr(VI) in Portland Cement. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Sharma D. K, Sharma R. Use of Variamine Blue dye in Spectrophotometric determination of Water Soluble Cr(VI) in Portland Cement. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12936 |
Introduction
Chromium was present in raw materials used for the production of cement. In its +3 oxidation state, it is not harmful, but during production of cement, at high temperature this harmless form gets converted into toxic hexavalent form [1]. Hexavalent chromium is water soluble and leached out during hydration of cement. Leaching of Cr(VI) becomes a health hazard for the workers at construction field because it can cause cancer, mutations and skin dermatitis [2-5]. Contact dermatitis is one of the major problems reported in the workers at construction sites, who worked with cement [6-9]. To control the toxicity of Cr(VI), European government has limited the chromium content to 2.0 ppm relative to the mass of cement [10]. Number of research showed that in India chromium level was found in between 12 to 33 ppm [11]. Keeping the toxicity of cement in mind, it becomes necessary to estimate the chromium content in cement used [12]. Several methods for determination of hexavalent chromium in cement samples are available in literature. Spectrographic method for the determination of hexavalent chromium using diphenylcarbazide (DPC) method [13-15], where extracted solution from cement was adjusted to final volume and acidified (pH 2.1 to 2.5). Then 0.25% solution of DPC was added. DPC causes reduction of Cr(VI) to Cr(III) by itself undergoing oxidation. Thus oxidized DPC and Cr(III) formed a magenta-colored complex. This concentration of complex was measured by the spectrophotometer with a maximum absorption at 540 nm, However, the matrix of cement-related materials is complicated and contains a variety of interacting cations such as Mn+2, Fe+2, Fe+3, and Ca+2 that may cause problems with the accurate determination of Cr(VI) using DPC methods [16]. Therefore, the estimation of Cr (VI) species in cement-related materials requires an additional effort.
An oxidizing agent (Na2S2O8) was implemented to remove the interfering effect of reducing agent (ferrous ion) and ortho phosphoric acid was used for the removal of interfering species (ferric ion) during detection by standard DPC method. These implementations had been taken in standard method, known as EN196-10 [17]. Some author proposed hyper technique and developed methods to determine chromium species in cement through HPLC-ICP-MS, FPLC-ETAAS, Wavelength-Dispersive X-ray Fluorescence Spectrometry, direct spectrophotometric determination of Cr(VI) in 1, 2, 5, 8 Tetrahydroxyanthraquinone (Quinalizarin), Catalytic adsorptive stripping voltammetry with DTPA and nitrate [18-22]. Although these advanced techniques can detect chromium in very low concentration with high accuracy, precision, and selectivity, but the cost is very high. On industrial level application of these techniques is not possible. Thus spectroscopic methods are still the most used techniques for the determination of Cr(VI) in cement extracts [23-25].
The most widely used method for determination of hexavalent chromium from cement extract is DPC, but it suffers serious interference from ions present in aqueous cement extract, high blank value and also formed complex (Cr+3 coordinated with diphenylcarbazone (DPCA)) is stable for 30 minutes [10]. Keeping these drawbacks in mind, in present work a facile, sensitive, accurate and reliable method for the determination of trace amounts of the chromium is used.
Experimental
Apparatus
A HACH Spectrophotometer (Made in USA) with 1 cm quartz cell was used for the absorbance measurements. A pH meter and conductometer was used to monitor the pH of the equilibrating solutions and conductivity of double distilled water. The pH meter was standardized using pH 4, 7, and 10 buffer solutions. A digital balance was used for weighing all the reagents.
Chemicals and Solutions
All chemicals used were of analytical reagent grade and doubly distilled water, were used for the preparation of aqueous solutions. All reagents used were of analytical reagent grade (A.R. grade). Variamine blue dye solution (0.05%) was prepared by dissolving 0.05 gram in 25 mL absolute alcohol and diluted to 100 mL with double distilled water. The 0.25% diphenylcarbazide (DPC) solution was prepared as per the procedure followed by 1.0 gram of diphenylcarbazide (DPC) was dissolved in 75 mL ethanol. To this was added 5 gram phthalic anhydride and 6 drops of concentrated H2SO4 and made up to 100 mL with ethanol. Standard Cr(VI) stock solution (1000 ppm) was prepared by dissolving 0.2829 gram of K2Cr2O7 in 100 mL of water. This stock solution was used for the preparation of working solutions. Sulfuric acid (1 M) was prepared by diluting 6.95 mL of stock H2SO4 to the mark in a 250 mL volumetric flask with water. A solution of potassium iodide (2.0%) was prepared by dissolving 2.0 gram potassium iodide in water and diluting it up to100 mL. A solution of sodium acetate (2M) was prepared by dissolving 16.407 gram sodium acetate in water and diluting the solution to 100 mL in a volumetric flask.
Cement Samples
The Ordinary Portland Cement (OPC, 43-Grade of ACC brand) used for this work, samples of ordinary Portland cement (OPC) were supplied by Oriental Structural Engineers Pvt. Ltd. Chemical composition of OPC has been carried out and data obtained are presented in Table 1.0, respectively.
Table 1 : Chemical composition of ordinary Portland cement (OPC-43 grade)
| Composition | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | Na2O | K2O | LOI | Others |
| % (w/w) | 63.5 | 21.70 | 5.84 | 3.50 | 2.00 | 1.10 | 0.35 | 0.10 | 0.5 | 0.30 |
Determination of water soluble Cr(VI) in cement samples
Aqueous cement extracts were prepared according to the European standard method (EN196-10) [17], weigh accurately 25.0 g of the cement sample in mixer with 25.0 mL of distilled water, mixing the mixture for 15 ± 1 minute intensively at 300 rpm. Immediately filter the suspension with vacuum filtration. A known amount of aliquot of cement sample was spiked with known amount of Cr(VI). Thus the determination of water soluble Cr(VI) in cement extract as well as in spiked cement extract samples by variamine blue (proposed method) as well asanalyzed according to the standard diphenylcarbazide method (reference method).
Variamine Blue method (proposed method)
An aliquot of cement samples solution with and without added Cr(VI) were taken in volumetric flasks. Potassium iodide (2%, 1 mL), sulphuric acid (2M, 1 mL) were added and mixture was gently shaken until the appearance of yellow color (iodine liberated). Then variamine blue (0.05% 0.5 mL) was added to it, follow by the addition of sodium acetate solution (2M, 2 mL). The contents were mixed well; the absorbance of the colored species was measured at 556 nm against the corresponding reagent blank (Scheme 1).
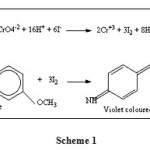 |
Scheme 1 Click here to View scheme |
Diphenyl carbazide method (Reference method)
To cement samples, 2.5 mL of 1, 5 dipenyl carbazide solutions and sulfuric acid (1:4) was added. The solution was then made upto 100 mL with double distilled water. DPC causes reduction of Cr(VI) to Cr(III) by itself undergoing oxidation. Thus DPCA and Cr(III) formed a magenta-colored complex (Scheme 2). Absorbance of all the solutions against blank was recorded at wavelength of 540 nm
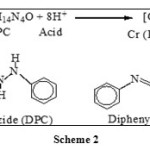 |
Scheme 2 Click here to View scheme |
Result and Discussion
Analytical data
The quantitative determination of soluble Cr(VI) in aqueous solutions was done by constructing a calibration plot. For the calibration curve, solution of concentration varying from 2-12 ppm with addition of all chemicals and reagents required for detection were made and their absorbance values were recorded. The plot of absorbance versus concentration was found to be linear till 12 ppm of soluble Cr(VI). A linear relationship between absorbance values and amount of soluble Cr(VI) suggests the use of the present method for quantitative determination of Cr(VI) in aqueous samples. The detection limits, quantification limit, slope and regression value are given in Table 2.
Table 2: Analytical parameters
|
Analytical parameters |
Proposed method (VB) |
Reference method (DPC) |
|
Detection limit (DL) |
0.05 |
0.04 |
|
Quantification limit (QL) |
0.18 |
0.13 |
|
Slope |
0.029 |
0.518 |
|
regression value |
0.998 |
0.996 |
|
Range (for linearity) |
2-12 |
0.05-1.0 |
|
Sensitivity |
Low |
High |
DL = 3 s/S, QL= 10 s/S where s is the standard deviation of the reagent blank (n=5) and S is the slope of the calibration curve
Validation of method
The percentage of recovery of various Cr(VI)-spiked solutions as well as their relative standard deviation, relative error and parametric test (t and f test) were also calculated to express the validation of proposed methods (results are given in table 3). Results were compared statistically for the validity of the proposed method. All the experiment were performed at least five times, and student’s t test and f test methodology were used for comparison between two different Cr(VI) determination method. The calculated t and f values were compared with test value at a proper degree of freedom, the results showed that both methods are reliable up to 15 mg/L solution.
Table 3: Validation test
|
Proposed Method (VB) |
Reference method (DPC) |
||||||||||
|
Sample |
Cr(VI) added (ppm) |
Cr(VI) found (ppm) |
RSD* (%) |
Relative error (%) |
Recovery (%) |
Cr(VI) found (ppm) |
RSD (%) |
Relative error (%) |
Recovery (%) |
Ta test |
Fb Test |
|
OPC NIST |
5 |
4.55± 1.2 |
9.87 |
-9 |
91 |
4.93± 1.2 |
14.59 |
-1.4 |
98.6 |
0.13 |
0.33 |
|
10 |
9.78± 0.5 |
8.87 |
-2.2 |
97.8 |
10.0± 0.9 |
10.59 |
0.2 |
100.2 |
0.39 |
0.64 |
|
|
15 |
15.01± 1.1 |
4.43 |
0.07 |
100.06 |
15.05± 1.4 |
10.65 |
0.33 |
100.33 |
0.85 |
0.10 |
|
*Relative standard deviation , Mean ± standard deviation (n = 5). a Tabulated t-value for 8 degrees of freedom at 5% level of significance is 2.306. b Tabulated F-value for (4,4) degrees of freedom at P (0.95) is 6.39.
Determination of Soluble Cr(VI) in Cement samples
The proposed method was applied to the quantitative determination of water soluble Cr(VI) in cement samples, the results are presented in Table 4. A variation in results was obtained in detection by standard method and present method (Figure 1). High concentration of Cr(VI) by DPC method may be due to presence of many interfering ions, because it was already mentioned in literature that DPC reagent was very sensitive to produce color [16].
Table 4: Water soluble Cr(VI) in Ordinary Portland cement samples
|
S.No |
Sample ID |
Date of manufacturing |
Soluble hexavalent chromium (ppm) Average (n=5 replicate) ± Standard deviation |
|||
|
Weak No./Year |
VB |
RSD |
DPC |
RSD |
||
|
1 |
OPC NIST |
– |
0.98±0.03 |
3.06 |
1.09±0.02 |
1.8 |
|
2 |
OPC 1 |
40/2014 |
31±0.5 |
1.6 |
33±1.5 |
4.5 |
|
3 |
OPC 2 |
42/2014 |
27±0.9 |
3.3 |
30±1.9 |
6.3 |
|
4 |
OPC 3 |
01/2015 |
28±0.7 |
2.5 |
34±2.5 |
7.4 |
|
5 |
OPC 4 |
04/2015 |
32±0.5 |
1.6 |
33±2.0 |
6.06 |
|
6 |
OPC 5 |
05/2015 |
36±0.8 |
2.2 |
40±1.2 |
3.0 |
RSD: Relative standard deviation
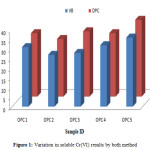 |
Figure 1: Variation in soluble Cr(VI) results by both method Click here to View figure |
Effects of Ions on the Variamine Blue Spectroscopic method
An additional effort was made to investigate the effect of various common ions on spectroscopic determination of 5 ppm soluble Cr(VI) from variamine blue method. For cations like Na+ and K+, no significant effects were observed up to 1,000 mg/L of each ion. Aluminum (Al3+) and magnesium ions (Mg2+) may interfere with the Cr(VI) determination when the concentration is higher than 500 mg/L. Common anions, such as nitrate (NO3–) and chloride (Cl–), did not affect up to 750 mg/L, and no effects were observed for sulfate (SO42-) and carbonate (CO32-) up to 1200 mg/L. Effect of ferric ion (25 ppm) may be considerable, but can be minimized by addition of 5.0 mL of orthophosphoric acid.
Conclusions
The chromium concentration estimated by DPC method was found to be more than variamine blue method. Comparatively high concentration of chromium in DPC method may be attributed to interference caused by various ions because of high colour sensitivity of DPC. Since, the interference by other ions is less in present method, this method can be used for accurate detection of Cr(VI) in cement sample. High colour stability of Variamine blue dye added an additional feature to this reagent.
Acknowledgements
The authors wish to thanks Dr. N. K. Katyal, Ex Incharge “Analytical Lab Service” National Council for Cement and Building Materials, Ballabgarh (Haryana), India, For providing the facilities for analysis of cement samples.
References
- Roskovic R., Oslakovic I.S., Radic J., Serdar M, Effects of chromium (VI) reducing agents in cement on corrosion of reinforcing steel, Cement and Concrete Composites, 2011, 33, 1020-1025.
- Hills L and Johansen VC “Hexavalent Chromium in Cement Manufacturing:Literature Review”, Portland cement Association, Skokie,. 2007
- Wetterhahn KE and Hamilton JW, “Molecular basis of hexavalent chromium carcinogenicity”, effect on gene expression. Sci. Total Environ, 1989.,86(1-2), 113.
- Witmer CM, Park HS and Shupack SI, “Mutagencity and desorption ofchromium”, Sci. Total Environ., 1989.,86(1-2), 131-148.
- Katz SA and Salem H “The Biological and Environmental Chemistryof Chromium.”,VCH New York:, 1994, 97–103.
- Jager H, Pelloni E, “Tests epicutanes aux bichromates, positifs dansl’eczema au ciment”. Dermatologica 1950, 100: 207.
- Halbert AR, Gebauer KA, Wall LM, “Prognosis of occupational chromate dermatitis”. Contact dermatitis 1992.,27, 214- 219.
- Geier J and Schnuch A “A comparison of contact allergies among construction and non construction workers attending contact dermatitis clinics in Germany”. Amer J Contact Derm 1995, 6, 86-94.
- Guo YL, Wang BJ, Yeh KC, Wang JC, Kao HH, Wang MT, Shih HC and Chen CJ “Dramatises in cement workers in Southern Taiwan”, Contact Dermatitis 1999, 40, 1-7.
- CSTEE (The Scientific Committee on Toxicity, Ecotoxicity and the Environment) “Risk to Health from Chromium Hexavalent in Cement”,CSTEE, Brussels. (2002)
- Sharma R, Sharma DK, Katyal, N.K. Study of total chromium and water soluble chromium in Indian cement samples. International Journal of Advancements in Research & Technology. 2014; 3 (2): 1-5.
- Unceta N, Séby F, Malherbe J and Donard OFX, “Chromium speciation in solid matrices and regulation: a review”, Anal Bioanal Chem 2010.,397: 1097–1111.
- G. Robert. M. S. Keenan, B. Vernon Perone, Hexavalent Chromium and Total Chromium, American Industrial Hygiene Association Quarterly. 1957., 18 (3) 231-234.
- Bose. M, The reaction of chromate with diphenylcarbazide, Analytica Chimica Acta, 1954.,10, , 201–208.
- Pflaum. R. T, Howick L. C, The chromium-diphenylcarbazide reaction, Journal of the American Chemical Society, 1956 78 (19), , 4862–4866.
- Narayana B and Cherian T Rapid Spectrophotometric Determinationof Trace Amounts of Chromium Using Variamine Blue as a ChromogenicReagent. J. Braz. Chem 2005., 16 (2):197-201.
- European Committee for Standardization, EN196-10 Methods of testing cement – Part 10: Determination of the water soluble chromium (VI) content of cement, Brussels. (2006).
- S.S. Potgieter, N. Panichev, J.H. Potgieter, S. Panicheva, Determination of hexavalent chromium in South African cements and cement-related materials with electrothermal atomic absorption spectrometry, Cement and Concrete Research. 2003., 33 1589–1593.
- J. Scancar, R. Milacic, F. Séby, Donard OFX, Determination of hexavalent chromium in cement by the use of HPLC-ICP-MS, FPLC-ETAAS, spectrophotometer and selective extraction techniques, J Anal At Spectrom. 2005.,20., 871–875.
- Eva Margui, Determination of Water-Soluble Hexavalent Chromium in Clinker Samples by Wavelength-Dispersive X-ray Fluorescence Spectrometry After Concentration in Activated Layers, Applied spectroscopy, 2010., 64 547-551.
- K. A. Idriss, H. Sedaira, S. Dardeery, Spectrophotometric Determination of Water-Soluble Hexavalent Chromium and Determination of Total Hexavalent Chromium Content of Portland Cement in the Presence of Iron (III) and Titanium (IV) Using Derivative Ratio Spectrophotometry, American Journal of Analytical Chemistry, 2013, 4., 653-660.
- Pawel Zajac and et al (2014), Volta metric methods for chromium(vi) determination in extracts from coal fly ash and cement, YISAC 2014, June 25–28, 2014, Pardubice, Czech Republic, Chem. Listy 108, 197–203 (2014).
- D. K. Sharma, R. Sharma, Indian Journal of Science and Technology. 2015.,.8(11)
- Sharma, R.; Sharma, D. K.; EBAS, Proceedings, Elsevier, 2014, 978-93-5107-313-0.
- Sharma, D. K.; Sharma, R.; Orient. J. Chem, 2015, 31(1), 519-526.

This work is licensed under a Creative Commons Attribution 4.0 International License.









