Adsorption of methylene blue onto artichoke waste
Ouzidan Fatima, El Kouali Mhamed, Talbi Mohamed and Atmani Rachid
Research Laboratory of Analytical Chemistry and Physical Chemistry of Materials, Faculty of sciences Ben M’sik, University Hassan II - Casablanca, Morocco. Corresponding Author Email: Ouzidan.fatima86@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310422
Article Received on :
Article Accepted on :
Article Published : 09 Dec 2015
The objective of this study is the removal of methylene blue dye by adsorption on activated carbon prepared from artichoke waste. Adsorption tests showed that the equilibrium is established after 100 minutes. Different experimental factors were analyzed: the size, mass of adsorbent and the initial concentration of methylene blue. The results of the experiment showed that the adsorption of methylene blue dye on the artichoke waste depends on the particle size and the initial concentration of dye. The adsorption capacity was determined using the Langmuir and Freundlich isotherms. The adsorption kinetics of methylene blue was studied using the equations of pseudo-first-order and pseudo-second- order. The adsorption of methylene blue dye artichoke waste can be described by a kinetic pseudo-second-order.
KEYWORDS:Adsorption; activated carbon; artichoke waste; methylene blue; kinetics
Download this article as:| Copy the following to cite this article: Fatima O, Mhamed E. K, Mohamed T, Rachid A. Adsorption of methylene blue onto artichoke waste. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Fatima O, Mhamed E. K, Mohamed T, Rachid A. Adsorption of methylene blue onto artichoke waste. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13186 |
Introduction
Textile industries release large quantities of wastewater at risk of toxicity. The wastewater has low biodegradability, which makes biological treatments impractical agricultural byproducts, and this is a source of environmental degradation. Adsorption is a promising technique due to the simplicity of its use as well as its low cost compared to other applications in the bleaching process cost. This is particularly true if the adsorbent is cheap and readily available.
Several adsorbents are used for the treatment of these wastewaters such as orange peels [1] and banana [2], eggshells [3}, sawdust, date seeds [4], clays [5] and tea waste [6].
In our study, we tried to evaluate the effectiveness of removal of methylene blue on waste from food; artichoke waste.
Materials and Methods
The adsorbent used in this work is artichoke waste which was washed, then dried in an oven at 100 ° C for 24 hours. It was crushed, then carbonized at 650° C for one hour and a half, then sieved to obtain two different types of fractions: the first fraction is characterized by a diameter smaller than 80 microns (d <80 microns), and the second fraction with a diameter of between 80 microns and 2 mm (80 microns <d <2mm).
The dye in this study was considered the methylene blue; Figure 1 shows the molecular structure. The stock solution of 100 mg/ l was prepared by dissolving 100 mg of the dye in distilled water. The colored solutions of different concentrations used in this study were prepared by the process of dilution using distilled water.
With drawls over time made it possible to monitor the concentration of dye remaining in solution. The residual concentration was determined from a calibration curve, analyzed by a UV / visible spectrometer (Shimadzu UV mini-1240).
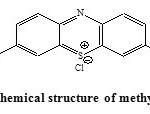 |
Figure 1: Chemical structure of methylene blue. |
Results and Discussion
Effect of Particle Size
Figure 2 shows the influence of the particle size of the adsorbent on the adsorption of methylene blue. When we used two types of fraction, the first fraction with the particle size less than 80 microns, and the second fractions between 80 microns and 2 mm, it shows that the adsorption is fast and relatively important for the fine particles (G ≤80 microns). This could be explained by the fact that the importance of adsorption depends on extern surface of particles; the smaller the size of particles is, the more important the supplied surfaces of exchanges are favoring a big spread of the dye to the adsorbent. We take into account this result and we are going to continue to work on this size.
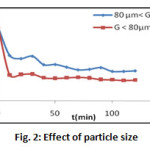 |
Figure 2: Effect of particle size Click here to View figure |
Effect of mass
The tests were carried out by stirring 100 ml of dye solution at 10 mg / l, with different masses of the support, in beakers of 250 ml under a constant agitation of 800 rev / min. The curve of the figure below shows the variation of the quantity of adsorption according to time
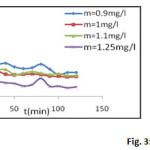 |
Figure 3: Effect of mass |
Effect of concentration
To study the influence of the concentration of the adsorbent material on the fixation of the dyes, tests were conducted with variable concentrations and other constant parameters. The results are shown in Figure 4.
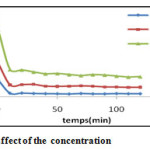 |
Figure 4: Effect of the concentration Click here to View figure |
Modeling of adsorption kinetics
The first order model is generally expressed as:
![]()
This model suggests the existence of a Chimisorption [7], an exchange of electrons between such a molecule of adsorbate and adsorbent solid. It is represented by the following formula:
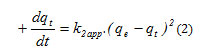
K2: constant of speed of adsorption of the model pseudo second order (g.mg-1.min-1)
qt: The adsorption capacity at the time t.
qe: The adsorption capacity at equilibrium.
The integration of equation (2) gives:
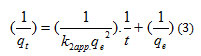
The values for the constants K1, K2 and the factors of correlation are grouped on Table1 and table 2.
Table 1: The parameters of the kinetic model of the first order
|
K1 |
qe |
R2 |
|
| m=1g/l;C0=5mg/l |
0,0117 |
0,1525 |
0,2422 |
| m=1g/l ;C0=10mg/l |
0,0285 |
1,1134 |
0,9788 |
| m=1g/l ;C0=20mg/l |
0,0282 |
3,1474 |
0,976 |
| m=0,9g/l ;C0=10mg/l |
0,0061 |
1,7130 |
0,6337 |
| m=1,1g/l ;C0=10mg/l |
0,0367 |
2,1393 |
0,8101 |
| m=1,25g/l ;C0=10mg/l |
0,0184 |
1,2628 |
0,9981 |
Table 2: The parameters of the kinetic model of the second order
|
K2 |
qe |
R2 |
|
| m=1g/l;C0=5mg/l |
0,6182 |
0,6182 |
0,8248 |
| m=1g/l ;C0=10mg/l |
0,1185 |
0,1185 |
0,9856 |
| m=1g/l ;C0=20mg/l |
0,1269 |
0,1269 |
0,9835 |
| m=0,9g/l ;C0=10mg/l |
0,0457 |
0,0457 |
0,8875 |
| m=1,1g/l ;C0=10mg/l |
0,0522 |
0,0522 |
0,982 |
| m=1,25g/l ;C0=10mg/l |
0,0792 |
0,0792 |
0,9801 |
Isotherm of adsorption
Model of Langmuir
This model is defined by a maximum capacity of adsorption that has been bound to the cover of surface sites by a monolayer. The importance of the Langmuir Isotherm is that it can be in theory applied to a perfectly uniform surface, and when there is no interaction between the adsorbed molecules [8]. The relation that characterizes this model is as follows:
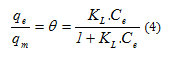
q: capacity of adsorption in mg/ l.
qm: Maximum capacity of adsorption in mg/ l.
K: The equilibrium constant of adsorption of Langmuir in l/mg.
Ce: Concentration of the adsorbate at adsorption equilibrium.
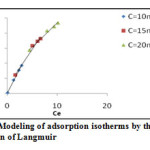 |
Figure 5: Modeling of adsorption isotherms by the equation of Langmuir Click here to View figure |
Table 3: Parameters of Langmuir model
|
C0(mg/l) |
qm |
KL |
R2 |
RL |
|
10 |
7,9617 | 13,0838 | 0,9972 | 0,0075 |
|
15 |
10,3092 | 0,9719 | 0,9813 | 0,064 |
|
20 |
15,9489 | 0,2064 | 0,9884 | 0,195 |
The viability of the adsorption can be defined from the factor of separation RL:
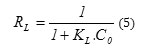
RL>1 conditions are unfavorable adsorption;
RL<1 conditions are favorable
adsorption;
RL =0adsorptionis irreversible.
The factor of separation is less than 1 for all concentrations, thus the adsorption is favorable.
Model of Freundlich
This model is used in the case of possible formation of more than one monolayer of adsorption on the surface and the sites are heterogeneous with different energies of fixation. [9]
The relationship that characterizes this model is given in the form:
Q: Quantity adsorbed by gram of the solid.
Ce: Concentration of the Adsorbate at adsorption equilibrium.
Kf and nf: Freundlich constants characteristic of a given efficiency vis-à-vis a given solute adsorbent
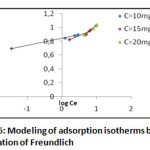 |
Figure 6: Modeling of adsorption isotherms by the equation of Freundlich |
Table 4: Parameters of Freundlich model
|
C0 (mg/l) |
qm |
KF |
n |
R2 |
|
10 |
88,517 |
6,995 |
0,1022 | 0,9963 |
|
15 |
146,422 |
5,950 |
0,215 | 0,9317 |
|
20 |
215,565 |
4,168 |
0,4126 | 0,9916 |
Conclusion
The study of the mechanisms of the adsorption of the methylene blue on the waste of the artichoke has been the subject of this work. The results related to the kinetics and isotherms of adsorption have been exploited to explain the method of fixation of the dye on the adsorbent. The study of the influence of the mass and the initial concentration on the kinetics showed that the process of adsorption follows the model of pseudo-second order. The adsorption capacity of a mass of the waste of artichoke increases with the increase of the initial concentration of the dye in the solution. The model of Langmuir expresses the type of adsorption; the dye molecules are adsorbed in a monolayer.
References
- Mezenner, Y.; Bensaadi, N.; Lagha, H. ; Bensmaili, A. ; “Etude de l’adsorption d’une mixture de composes biorecacitrants en milieu aqueux”, 2013.
- Khalfoui, A.; “Etude expérimentale de l’élimination de polluants organique et Inorganique par adsorption sur des matériaux naturels : Application aux peaux d’orange et de banane”, doctoral dissertation, Algérie, 2012.
- Ounas, A.; Bergach, N.; Ennaciri, K.; Yaacoubi, A. ; Bacaoui, A.; “Préparation des charbons actifs a partir des déchets de l’industrie oléicole”.
- Bouchemal, N.; Merzougui, Z.; Addoun, F.; “Adsorption en milieux aqueux de deux colorants sur charbons actifs à base de noyaux de datte ”, J. Soc. Alger. Chim, (21), 2011, 1-14.
- Errais, E.; “Réactivité de surface d’argiles naturelles ; Etude de l’adsorption de colorant anionique”, doctoral dissertation, Strasbourg, 2011.
- Reffas, A.; “Etude de l’adsorption de colorants organiques (rouge nylosan et blen de méthylène) sur des charbons actifs préparés à partir du marc de café”, doctoral dissertation, Chambéry, 2010.
- Tavis, C.C.; Etnier, E.L.; “A survey of sorption relationships for reactive solutes”. Journal of Environmental Quality, 1981,10(1), 8-17.
- Gherbi, N.;“Etude expérimentale et identification du processus de rétention des cations métallique par des matériaux naturels”, doctoral dissertation, Algérie, 2008.
- Hadj Salah, N.; “Etude de la dégradation photocatalytique de polluants organique en présence de dioxyde de titane; en suspension aqueuse et en lit fixe”, doctoral dissertation, Grenoble, 2012.

This work is licensed under a Creative Commons Attribution 4.0 International License.









