A Quantitative Assessment of the Nucleophilicity of Cytosine in Aqueous Solution by Polarography Complemented by Hydrodynamic Voltammetry.
Willayat Bashir, V. T. Borkar and V. T. Dangat*
Department of Chemistry, Nowrosjee Wadia College, Pune 411001 India. Corresponding Author E-mail: vijaydangat@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310474
Article Received on :
Article Accepted on :
Article Published : 05 Nov 2015
The nucleophilicity of the nucleobase cytosine has been investigated by polarographic studies complemented by fast reaction kinetic data .Increasing half-wave potentials with pH are observed for the bio-molecule using the dropping mercury electrode (DME).The bromination of the nucleobase in aqueous solution is found to be a rapid reaction necessitating a special technique to follow the kinetics .Herein we have adopted the hydrodynamic voltammetry technique. The reaction is found to be of the second order, the specific reaction rate of which increases with pH .The complementary data in the two–pronged study reveals the reactivity of the nucleobase in this aromatic electrophilic substitution reaction in aqueous medium on a quantitative scaffold.
KEYWORDS:Cytosine, RPE, SCE, Polarography, Hydrodynamic Voltammetry
Download this article as:| Copy the following to cite this article: Bashir W, Borkar V. T, Dangat V. T. A Quantitative Assessment of the Nucleophilicity of Cytosine in Aqueous Solution by Polarography Complemented by Hydrodynamic Voltammetry. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Bashir W, Borkar V. T, Dangat V. T. A Quantitative Assessment of the Nucleophilicity of Cytosine in Aqueous Solution by Polarography Complemented by Hydrodynamic Voltammetry. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12203 |
Introduction
Nucleic acids are important components of living cells as they store and transmit hereditary information from generation to generation 1-2. Nucleic acids are polymers of polynucleotide chains3,4 and each monomer in the nucleotide in turn consists of a sugar phosphate backbone and a heterocyclic nitrogenous base which is either a purine (adenine, guanine) or pyrimidine(cytosine, thymine and uracil) 4,5 . These nitrogenous bases are important as per their function in genetic coding 5,6 It is important to study their functions and behaviour in living systems because of their role in carcinogenesis, mutagenesis and atrophy of cells13. Attempts to study them by various techniques are in progress. Of these,voltammetry has an important role7,8,9. Nucleic acids were first determined by Palecek using the Polarographic technique by the dropping mercury electrode (DME) 10. It is seen that only a few of the nitrogenous bases were electro-reducible at the (DME) 11 Presently we have adopted a two-fold approach to explore the reactivity of some nucleobases at the (DME) versus the saturated calomel electrode (SCE) and have studied the effect of pH on the redox propensity of these bases.
Further, we have studied the kinetics of bromination of cytosine using a special method –hydrodynamic voltammetry12,13 which is a voltammetric method in which a rotating platinum electrode is used to monitor the fall in concentration of molecular bromine in aqueous medium. This special technique was necessitated in view of the rapidity of the bromination reaction of the nucleobase.
Halogenations of these nucleobases is of significance in drug chemistry because halogenated heterocyclic nucleobases have an important role as antiviral, antibacterial, anticancer drugs either singly or as a motif of precursors of drug molecules14,15,16 Such reactions have been studied in the past using different methods in different reaction mediums but studies in purely aqueous medium at 7 pH without any catalyst seem lacking presumably due to the rapidity of these reactions under these conditions.
Experimental
Materials and Instruments
A R Grade cytosine was purchased from Himedia India. Doubly -distilled water was used for the preparation phosphate, acetate and borate buffer solutions and cytosine. D.C polarograph from Elico Model L1-10T and Digital pH meter from Equiptronics Model EQ-610 was used.
Polarographic measurements
5×10-5 M solution of cytosine was prepared and buffers were added to it along with100 fold KNO3, as the supporting electrolyte. Freshly prepared 0.004% gelatine was also added.25 ml of each solution was placed in a glass beaker covered with a glass lid and nitrogen gas were passed through the solution for 10 minutes to remove the dissolved oxygen from the solution which was then transferred to the polarographic cell with three electrode system having DME cathode, SCE anode and platinum electrode as the reference electrode. The drop rate was regulated to 22 drops per minute. 100-1400 mV potential was applied to this electrode system and the diffusion limited nano current was measured. Cytosine was reduced at the dropping mercury electrode. The instrument sensitivity was 100 nA/division and span rate 150 mV/minute. The polarogram obtained indicating the half-wave potential for the cytosine at pH 6 in aqueous medium is typically shown.
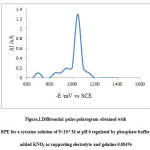 |
Figure 1: Differential pulse polarogram obtained with RPE for a cytosine solution of 5×10-4 M at pH 6 regulated by phosphate buffer, added KNO3 as supporting electrolyte and gelatine 0.004% |
Polarographic Results
The half-wave reduction potential for cytosine was observed to be 1100 mV. There is a pronounced effect of pH on the half-wave potential of the nucleobase. The polarogram of the reducing species was obtained at different pH values in the aqueous solution it was observed that with increase in the pH of the solution the half-wave potential shifts to more negative values as indicated by the graph. The polarograms were obtained between the pH range 4 – 7 by using acetate buffer for lower pH and phosphate buffer for higher pH values.
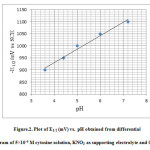 |
Figure 2: Plot of E1/2 (mV) vs. pH obtained from differential pulse polarogram of 5×10-4 M cytosine solution, KNO3 as supporting electrolyte and 0.004% gelatine. |
After completing the effect of pH we determined the sensitivity of the DME to detect the concentration of cytosine in aqueous medium and this value was found to be 2.5×10-4 M Below this concentration it was not possible to detect any reduction peak of the cytosine in the aqueous medium. This corresponds to the sensitivity value reported in literature but for glassy carbon electrode.
Halogenation Of Cytosine
Halogenations of cytosine in aqueous medium by molecular bromine is fast reaction . To study the kinetics of this reaction we have employed a special technique hydrodynamic voltammetry 12,13 17.
Materials and instruments
Cytosine base was purchased from Himedia India, molecular Br2 was prepared in laboratory by diluting concentrated bromine in doubly-distilled water. The concentration was determined by iodometry. KNO3, moving coil galvanometer, potentiometer, platinum electrode rotating at 600 rpm by a synchronous A.C motor and buffers to maintain the pH were also used.
Calibration of the diffusion current
The diffusion current was calibrated by preparing different concentrations of Br2 in water with added buffers and the supporting electrolyte .These solutions were kept in the reaction cell and the galvanometer deflection were observed.
Kinetic measurements
3×10-5 M solution of both Br2 and cytosine with the added buffer to maintain pH at 7 and 100 fold KNO3 as supporting electrolyte were kept in thermostat in two different stoppered bottles. 50 ml of Br2 solution was transferred to reaction vessel of 250 ml capacity kept in the same thermostat. The galvanometer deflection was set to around 40 cm by the shunt. Then 50 ml of cytosine solution was added to the reaction mixture and immediately a stop watch was started the fall in the concentration of Br2 in terms of the galvanometer deflection was observed at every 10 seconds. This was repeated at different temperatures also. The graph of [Br2 ]-1versus time in seconds was plotted the experiment was repeated at 6.8 pH and 7.2 pH.
Table 1: Kinetics of bromination of cytosine by molecular bromine at 280C in aqueous medium.
|
Time /s |
Diffusion current /cm |
[Br2]/10-6M |
[Br2]-1/104M |
|
10 |
13 |
9.75 |
10.25 |
|
20 |
11.5 |
8.6 |
11.62 |
|
30 |
10.5 |
7.87 |
12.70 |
|
40 |
9.6 |
7.2 |
13.88 |
|
50 |
9 |
6.93 |
14.43 |
|
60 |
8.5 |
6.5 |
15.38 |
 |
Figure 3: Kinetics of bromination of cytosine by molecular bromine at various temperatures.
|
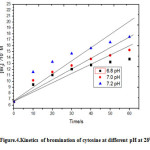 |
Figure 4: Kinetics of bromination of cytosine at different pH at 280C. Click here to View figure |
Table 2: The variation of specific reaction rates of bromination of cytosine in aqueous medium at different temperatures.
|
Temperature/K |
T-1/10-3K-1 |
k/M-1s-1 |
Log k |
|
292.15 |
3.42 |
869 |
2.93 |
|
296.15 |
3.37 |
1087 |
3.03 |
|
301.15 |
3.32 |
1236 |
3.09 |
|
304.15 |
3.28 |
1625 |
3.21 |
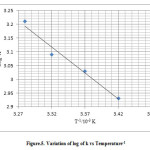 |
Figure 5: Variation of log of k vs Temperature-1 Click here to View figure |
Halogenation Results
The graph between [Br2]-1 and time in seconds was a straight line hence the reaction was concluded to follow second order kinetics8 .The specific reaction rate and other reaction parameters at 280C and pH 7 were calculated from the Arrhenius plot The mechanism of the reaction may be proposed as .
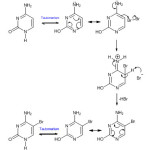 |
Scheme Click here to View scheme |
Table 3: Kinetic parameters of bromination of cytosine in aqueous medium at 280C and pH 7.
|
Kinetic parameter |
Value |
|
Specific reaction rate (M-1s-1) |
1236 |
|
Activation energy (kJmol-1) |
36.16 |
|
Frequency factor(M-1s-1) |
2.33×109 |
Conclusion
The polarographic half-wave carried out in the range 4 to 7 pH elucidates that at higher pH the half-wave reduction potential shifts to more negative potentials for aqueous cytosine solutions. This indicates greater nucleophilicity of the nucleobase at greater pH values implying that the nucleobase undergoes more rapid electrophilic substitution at greater pH values . This fact is borne out complementarily in the rapid kinetic study by hydrodynamic voltammetry wherein the bromination of cytosine in aqueous medium at 7.2 pH proceeds faster than at 7 pH which in turn proceeds faster than at 6.8 pH .The nucleobase at 7.2 pH is as expected more reactive in the electrophilic substitution bromination reaction than at a lower pH.
Acknowledgment
The authors are thankful to the Modern Education Society Pune, for providing the necessary facilities at the Post-graduate Department of Chemistry, Nowrosjee Wadia College, for carrying out this research work.
Refrences
- Strickberger, M. W,Genetics, third ed. Pearson Education,(1985).
- Roy,R.N.,.Heredity,Genetics and Genetical, Diseases, New Central Book Agency.London,(2011).
- Jain, J.L., Sunjay,Jain., Natin,Jain., Fundamentals of Biochemistry, S. Chand, (2005).
- Michael, M.Cox, David, L.Nelson,. Lehninger Principles of Biochemistry, fifth ed. W.H.Freeman and Company. New York,(2008).
- De Robertis, E.D.P., De Robertis.Jr, E.M.F.,.Cell and Molecular Biology, eight ed. Lippincott Williams and Wilkins,(2001).
- Oliveira-Brett, A.M., Piedade, J.A.P., Silva, L.A., Diculescu, V.C., Analytical Biochemistry.2004, 332, 321-329.
- Libuse,Trnkova.,Jiri,Friml.,Oldrich,Pracka.,.Bioelectrochemistry.2001,54,131-136.
- Zeinab,P.,Ali,N., Orient. J. Chem.2014,30(1),219-227.
- Shaikh,A,A.,Ehsan,M.Q.,Ahmad,S,A.,Ahmad,M,G.,Hussam,A.,Khan,A,H.,Orient. J. Chem.2014,20(3),
- Palecek, E., Talanta. 2002.56, 809-819
- James,W.Webb.,Borek,Janik.,Philip,J,Elving.,JACS. 1973,95,991-1003.
- Dangat, V.T., Bonde, S.L., Broker, V.T., Maske, V.T., Res.J.Chem.Sci. 2012,2, 75-78.
- Bonde, S.L., Dangat, V.T., Bhadane, R.P., Joshi, V.S., Int.J.Chem.Kinet.2013,45, 355-362.
- Sajik,H.,Iida,Y.,Ikawa,K.,Sawana,Y.,Monguchi,Y.,Kitude,Y.,Maki,Y.,Ious,H.,Hirota,K.,2.Molecule.17,6519-6546
- Lee,H.H.et.al.,.Bioorg.Med.Lett. 1999, 9,241-244.
- Leena,M.Patil.,Datta,E.Ponde.,Shriniwas,D.Samant., Heteroletters. 2014,4,559-564.
- Rao, T.S, Mali. S.I., Dangat, V.T., Tetrahedron.1978,34,205-208.
- Libuse,Trnkova.,Frantisek,Jalen.,Irena,Postbieglova.,.Electroanalysis.2002,15,1529-1535.
- Jai,Y.C.,Shen,M.C.,Wen,H.W.,Ying,L.,Shauh,D.Y.,.Int.J.Electrochem.Sci.2013,8,3963-3973.
- Oliveira-Brett, A.M., Diculescu, V., Piedade, J.A.P., Bioelectrochemistry.2002,55, 61-62.
- Oliveira-Brett, A.M, Serrano, S.H.P., J.Braz.Chem.Soc. 1995.6, 97-100.
- Palecek, E., Jelen, F, Trnkova;Gen.Physiol.Biophys.1986,5, 315-319.

This work is licensed under a Creative Commons Attribution 4.0 International License.









