A one-pot synthesis of functionalized azadienes from 2-hydroxypyridine, activated acetylenes and alkyl isocyanides
Elnaz Ghasemi and Issa Yavari*
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran 14515-775, Iran.
DOI : http://dx.doi.org/10.13005/ojc/310433
Article Received on :
Article Accepted on :
Article Published : 02 Nov 2015
A one-pot synthesis of dialkyl 2-[(alkylimino)(2-oxopyridin-1(2H)-yl)methyl]but-2-enedioate from alkyl isocyanides, dialkyl acetylenedicarboxylates, and 2-hydroxypyridine, in good yields, is described.
KEYWORDS:Azadienes; Alkyl isocanides; Activated acetylenes; 2-hydroxypyridine
Download this article as:| Copy the following to cite this article: Ghasemi E, Yavari I. A one-pot synthesis of functionalized azadienes from 2-hydroxypyridine, activated acetylenes and alkyl isocyanides. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Ghasemi E, Yavari I. A one-pot synthesis of functionalized azadienes from 2-hydroxypyridine, activated acetylenes and alkyl isocyanides. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12145 |
Introduction
Isocyanides are known to form zwitterions with activated acetylenic compounds such as dimethyl acetylenedicarboxylate [1]. Reaction between isocyanides, electron-deficient acetylenes and nucleophiles was first documented by Oakes in 1969 and 1973 [2, 3]. Such interesting and promising transformation was nearly forgotten until Yavari in 1996 extended its application to dibenzoylmethane as NuH [4]. Later on, more publications on such a reaction were published differing mostly in the nature of NuH used [5-10].
In continuation of our interest in the application of isocyanides in MCRs [11-14], we report an efficient synthesis of functionalized azadienes using isocyanides 1, dialkyl acetylenedicarboxylates 2, and 2-hydroxypyridine 3. This three-component reaction produces highly functionalized azadienes 4 in good yields (Scheme 1).
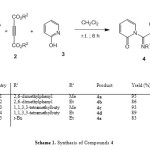 |
Scheme 1: Synthesis of Compounds 4 Click here to View scheme |
Experimental
All chemicals were obtained commercially and used without further purification. IR Spectra: Shimadzu-IR-460 spectrometer; bond positions in cm−1. 1H- and 13C-NMR Spectra: Bruker DRX-400 Avance instrument; in CDCl3 at 400 and 100 MHz, resp.; δ in ppm, J in Hz. MS: Finnigan-MAT-8430EI-MS mass spectrometer; at 70 eV; in m/z (rel. %). Elemental analyses: Vario EL III CHNOS elemental analyzer.
General procedure for the preparation of compounds 4.
To a stirred mixture of 3 (1 mmol) and acetylenic ester 2 (1 mmol) in CH2Cl2 (5 mL), was slowly added 1 (1 mmol) in CH2Cl2 (3 mL) at room temperature. After completion of the reaction [about 6 h; TLC (AcOEt/hexane 1:3) monitoring], the solvent was evaporated and the residue was purified by column chromatography [silica gel (230–240 mesh; Merck), hexane/AcOEt 2:1)] to give product.
Dimethyl 2-[(2,6-dimethylphenylimino)(2-oxopyridin-1(2H)-yl)methyl]but-2-enedioate (4a)
Yield: 0.35 g (95%). Yellow oil. IR (KBr): 1725 (C=O), 1660 (C=O), 1590 (C=N). 1H-NMR: 2.24 (6H, s, 2Me); 3.65 (s, MeO), 3.83 (s, MeO), 5.79 (s, CH), 6.02–7.97 (m, 7 CH). 13C-NMR: 19.2 (2Me); 55.2 (MeO); 55.9 (MeO); 105.2 (CH); 119.5 (CH); 120.4 (CH); 123.8 (CH); 128.1 (CH); 131.6 (2CH); 132.8 (C); 135.3 (2C); 139.9 (CH); 138.5(C); 163.2 (C=O); 168.1 (C=N); 171.1 (C=O); 173.4 (C=O). MS: 368 (17, M+), 309 (21), 274 (75), 169 (100), 105 (42), 94 (71), 59 (25). Anal. calc. for C20H20N2O5 (368.39): C 65.21, H 5.47, N 7.60; found: C 65.42, H 5.40, N 7.68.
Diethyl 2-[(2,6-dimethylphenylimino)(2-oxopyridin-1(2H)-yl)methyl]but-2-enedioate (4b)
Yield: 0.34 g (86%). Yellow oil. IR (KBr): 1728 (C=O), 1665 (C=O), 1574 (C=N). 1H-NMR: 1.26 (t, J = 7.2, Me); 1.45 (t, J = 7.2, Me); 2.15 (6H, s, 2Me); 4.24 (q, J = 7.2, CH2O); 4.37 (q, J = 7.2, CH2O); 5.85 (s, CH); 6.03–7.98 (m, 7 CH). 13C-NMR: 12.9 (Me), 13.1 (Me), 23.5 (2Me), 60.2 (CH2O), 61.2 (CH2O), 104.9 (CH); 120.1 (CH); 121.4 (CH); 124.3 (CH); 128.9 (CH); 132.6 (2CH); 134.8 (C); 136.3 (2C); 139.7 (CH); 138.7(C); 163.1 (C=O); 168.3 (C=N); 171.5 (C=O); 173.2 (C=O). MS: 396 (15, M+), 323 (20), 302 (77), 197 (100), 105 (40), 94 (68), 73(29). Anal. calc. for C22H24N2O5 (396.17): C 66.65, H 6.10, N 7.07; found: C 67.09, H 5.59, N 7.15.
Dimethyl 2-[(2,4,4-trimethylpentan-2-ylimino)(2-oxopyridin-1(2H)-yl)methyl]but-2-enedioate (4c)
Yield: 0.35 g (93%). Yellow oil. IR (KBr): 1735 (C=O), 1682 (C=O), 1590 (C=N). 1H-NMR: 1.24 (9 H, s, CMe3); 1.42 (6 H, s, CMe2); 1.60 (2 H, s, CH2); 3.71 (s, MeO); 3.79 (s, MeO); 5.74 (s, CH); 6.20–7.40 (m, 4 CH). 13C-NMR: 29.7 (CMe3); 30.9 (CMe2); 31.5 (CMe3); 51.7 (CH2); 55.2 (CMe2); 58.7 (MeO); 61.7 (MeO); 104.6 (CH); 119.4 (CH); 119.8 (CH); 136.4 (CH); 138.2 (C); 139.5 (CH); 163.6 (C=O); 168.5 (C=N); 171.3 (C=O); 173.7 (C=O). MS: 376 (15, M+), 317 (19), 282 (80), 169 (100), 113 (69), 94 (45), 59 (19). Anal. calc. for C20H28N2O5 (376.45): C 63.81, H 7.50, N 7.44; found: C 64.11, H 7.56, N 7.48.
Diethyl 2-[(2,4,4-trimethylpentan-2-ylimino)(2-oxopyridin-1(2H)-yl)methyl]but-2-enedioate (4d)
Yield: 0.36 g (89%). Yellow oil. IR (KBr): 1739 (C=O), 1676 (C=O), 1568 (C=N). 1H-NMR: 1.12 (t, J = 7.2, Me); 1.22 (t, J = 7.2, Me); 1.26 (9 H, s, CMe3); 1.45 (6 H, s, CMe2); 1.63 (2 H, s, CH2); 4.07 (q, J = 7.2, CH2O); 4.18 (q, J = 7.2, CH2O); 5.68 (s, CH); 6.07–7.54 (m, 4 CH). 13C-NMR: 13.1 (Me); 13.4 (Me); 29.9 (CMe3); 30.2 (CMe2); 32.5 (CMe3); 58.8 (CH2); 59.9 (CMe2); 61.0 (CH2O); 61.1 (CH2O); 104.5 (CH); 119.3 (CH); 119.5 (CH); 136.6 (CH); 138.8 (C); 139.8 (CH); 163.4 (C=O); 168.3 (C=N); 171.2 (C=O); 173.5 (C=O). MS: 404 (12, M+), 331 (17), 310 (80), 197 (100), 113 (72), 94 (40), 73 (22). Anal. calc. for C22H32N2O5 (404.51): C 65.32, H 7.97, N 6.93; found: C 65.63, H 8.03, N 6.94.
Diethyl 2-[(tert-butylimino)(2-oxopyridin-1(2H)-yl)methyl]but-2-enedioate (4e)
Yield: 0.29 g (83%). Yellow oil. IR (KBr): 1731 (C=O), 1673 (C=O), 1581 (C=N). 1H-NMR: 1.19 (9H, s, CMe3); 1.27 (t, J = 7.2, Me); 1.39 (t, J = 7.2, Me); 4.15 (q, J = 7.2, CH2O); 4.35 (q, J = 7.2, CH2O); 5.95 (s, CH); 6.22–7.45 (m, 4 CH). 13C-NMR: 12.9 (Me), 14.3 (Me), 30.8 (CMe3); 57.7 (CMe3) 60.6 (CH2O); 61.7 (CH2O); 109.5 (CH); 121.3 (CH); 126.5 (CH); 136.9 (CH); 137.8 (C); 139.2 (CH); 163.5 (C=O); 168.2 (C=N); 171.4 (C=O); 173.6 (C=O). MS: 348 (10, M+), 275 (17), 254 (82), 197 (100), 94 (42), 73 (68), 57 (22). Anal. calc. for C18H24N2O5 (348.40): C 62.05, H 6.94, N 8.04; found: C 62.19, H 6.86, N 8.01.
Results and Discussion
Thus, reaction of isocyanides 1, acetylenic esters 2, and 2-hydroxypyridine 3 proceeded spontaneously in CH2Cl2, and was completed within a few hours. The 1H- and 13C-NMR spectra of the crude products clearly indicated the formation of dialkyl 2-[(alkylimino)(2-oxopyridin-1(2H)-yl)methyl]but-2-enedioates 4. The structures of compounds 4a–4e were deduced from their IR, 1H-NMR, and 13C-NMR spectra. The 1H-NMR spectrum of 4a in CDCl3 showed a singlet for the two methyl of 2,6-dimethylphenyl (δ(H) 2.24), along with three singlets for methoxy (δ(H) 3.65 and 3.83) and methine (δ(H) 5.79) H-atoms. Characteristic multiplets for the Ph H-atoms were observed at δ(H) 6.02-7.97. The 13C-NMR spectrum of 4a exhibited 17 resonances in agreement with the proposed structure. The mass spectrum of 4a displayed the molecular ion peak at m/z 368. The NMR spectra of 4b-4e were similar to those of 4a except for the substituents.
A possible mechanism for these transformations is proposed in Scheme 2. It is conceivable that the reaction involves the initial formation of the 1:1 zwitterionic intermediate 5 between the isocyanide 1 and acetylenic ester 2 (Scheme 1). Protonation of 5 by the acidic compound 3 leads to intermediate 6. Subsequent attack of the resulting nucleophile 7 on the positively charged ion 6 affords azadiene derivatives 4.
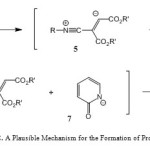 |
Scheme 2: A Plausible Mechanism for the Formation of Products 4. |
In conclusion, the three-component reaction of alkyl isocyanides, dialkyl acetylene dicarboxylates, and 2-hydroxypyridine provides a simple one-pot synthesis of stable functionalized azadienes. This procedure has the advantages of high yields and mild reaction conditions.
References
- Nair, V.; Rajesh, C.; Vinod, A. U.; et al. Acc. Chem. Res. 2003, 3, 899-907.
- Oakes, T. R.; David, H. G.; Nagel, F. J. J. Am. Chem. Soc. 1969, 91, 4761-4765.
- Oakes, T. R.; Donovan, D. J. J. Org. Chem. 1973, 38, 1319-1325.
- Yavari, I.; Davar-Panah, M.; Heydari, M.; et al. Monatsh. Chem. 1996, 127, 963-966.
- Yavari, I.; Djahaniani, H.; Nassiri, F. Monatsh. Chem. 2004, 135, 543-548.
- Shaabani, A.; Teimouri, M. B.; Arab-Ameri, S. Tetrahedron Lett. 2004, 45, 8409-8413.
- Bayat, M.; Imanieh, H.; Hosseininejad, E. Synthetic Commun. 2008, 15, 2567-2574.
- Yavari, I.; Hossaini, Z.; Sabbaghan, M. Tetrahedron Lett. 2008, 49, 844-846.
- Anary-Abbasinejad, M.; Anaraki-Ardakani, H.; Ghanea, F. Monatsh. Chem. 2009, 140, 397- 400
- Mohtat, B.; Djahaniani, H.; Khorrami, R.; et al. Synthetic Commun. 2011, 41, 784-791.
- Yavari, I.; Sanaeishoar, T.; Ghazvini, M.; et al. J. Sulfur Chem. 2010, 31, 169-176.
- Yavari, I.; Mirzaei, A.; Moradi, L.; et al. Tetrahedron Lett. 2010, 51, 396-398.
- Yavari, I.; Arab-Salmanabadi, S.; Aminkhani, A. Chinese Chem. Lett. 2012, 23, 49-52.
- Yavari, I.; Ghanbari, E.; Hosseinpour, R. Helv. Chim. Acta. 2014, 97, 1004-1008.

This work is licensed under a Creative Commons Attribution 4.0 International License.









