Tribromoisocyanuric acid (TBCA) as a mild and metal free catalyst for the acetylation and formylation of hydroxyl groups under solvent free conditions
Zahra Hekmatian1*,Ardeshir Khazaei2
1Department of Chemistry, Faculty of Science, Payam Noor University, Hamedan, Iran
2Faculty of Chemistry, Bu-Ali Sina University, P. O. Box 651783868, Hamedan,Iran. Corresponding Author Email: zahra.hekmatian88@yahoo.comDOI : http://dx.doi.org/10.13005/ojc/310335
Article Received on :
Article Accepted on :
Article Published : 31 Aug 2015
A convenient approach for acetylation and formylation of various types of alcohols and phenols with acetic anhydride and formic acid in the presence of Tribromoisocyanuric acid (TBCA) as catalyst is reported. The reactions were carried out under solvent-free condition and in good to high yields at room temperature. This present method is featured with relatively mild reaction conditions, simple operation, broad substrate scope, clean work-up, short reaction times, good to high yields, excellent selectivity and also avoids tedious purifications and the use of toxic reagents.
KEYWORDS:Tribromoisocyanuric acid (TBCA); Acetylation; Formylation; Alcohols; Phenols; Solvent-free
Download this article as:| Copy the following to cite this article: Hekmatian Z, Khazaei A. Tribromoisocyanuric acid (TBCA) as a mild and metal free catalyst for the acetylation and formylation of hydroxyl groups under solvent free conditions. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Hekmatian Z, Khazaei A. Tribromoisocyanuric acid (TBCA) as a mild and metal free catalyst for the acetylation and formylation of hydroxyl groups under solvent free conditions. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10476 |
Introduction
Protection-deprotection steps of active proticfunctional groups are frequently required in the synthesis of multifunctional compounds. The protection of alcohols and phenols by the formation of ester is one of the most important and widely used transformations in organic chemistry. Among the various protecting groups which have reported for the hydroxyl function, the acetyl is the most common because of its ease of formation as well as mild conditions for deprotection. Also,O-formylation is an important process in organic synthesis, because the formate esters serve as a useful synthetic reagent and intermediate (1-3). Generally, esters are synthesized through the reaction of alcohols with carboxylic acids or acylating/formylating agents, or by transesterification (4-6).
Acylation of alcohols with anhydrides is one of the most routinely used transformations in organic synthesis. A variety of procedures using homogeneous or heterogeneous catalyst such as ferric perchlorate adsorbedon silica-gel (7), MoO3@Al2O3 (8), phosphomolybdic acid (9), bismuth(III) salts (10), cerium polyoxometalate (11), InCl3 (12), Ce(OTf)3 (13), p-toluenesulfonic acid (14), alumina (15) have been reported for the acetylation of alcohols and phenols with acetic anhydride. Also a variety of procedures are routinely performed for the preparation of formyl derivatives including homogeneous or heterogeneous catalyst (16-22). However, in most cases these procedures suffer from one or more disadvantages such as stringent conditions, formation of undesirable or toxic by-products, hygroscopicity of the reagents, elevated reaction temperatures, longer reaction times, low yields of the desired products and expensive reagents. Thus there is a need for a mild, neutral and catalytically efficient alternative for the protection of hydroxyl groups as acetyl and formyl derivatives.
Material and Methods
Chemicals were purchased from Fluka, Merck and Aldrich Chemical Companies and were used as received, without further purification.
Preparation of tribromoisocyanuric acid (TBCA):
The cyanuric acid (5g, 38.5 mmol) was dissolved in a slight molar excess of chilled aqueous NaOH solution (3 M) at room temperature and the solution was transferred to a beaker. A solution of Br2 (3 mL, 58.4 mmol) in CCl4 (6 mL) was added drop wise to the reaction mixture with vigorous stirring at 0 °C. Immediately, an orange precipitate was formed. The resulting solid was isolated by a filtration and washed several times with cold distilled water and then, it was dried in a vacuum desiccator at room temperature for 18 h. Finally, an orange pale crystalline solid, tribromoisocyanuric acid, was obtained quantitatively.
Acetylation of benzyl alcohol using acetic anhydride catalyzed by tribromoisocyanuric acid
To a mixture of the benzyl alcohol (1 mmol) and acetic anhydride (3 mmol); 0.074 g tribromoisocyanuric acid (0.2 mmol) was added. The reaction mixture was stirred at room temperature for 12 min. After completion of the reaction (TLC), dichloromethane (10 mL) was added to the reaction mixture and filtered. The product was extracted with H2O (3×10 mL), and the organic layer was dried over anhydrous Na2SO4 salt. Then solvent was evaporated to dryness to afford the pure product.
Formylation of benzyl alcohol using formic acid catalyzed by tribromoisocyanuric acid:
To a mixture of the benzyl alcohol (1 mmol) and formic acid (3 mmol); 0.1 g tribromoisocyanuric acid (0.3 mmol) was added. The reaction mixture was stirred at room temperature for 5 min. After completion of the reaction (TLC), dichloromethane (10 mL) was added to the reaction mixture and filtered. The product was extracted with H2O (3×10 mL), and the organic layer was dried over anhydrous Na2SO4 salt. Evaporation of the solvent under reduced pressure to give the almost pure product.
Result and Discussion
In continuation of our studies on catalytic application of N-halo compounds as organocatalyst in organic chemistry (23–26), we decided to report the catalytic application of tribromoisocyanuric acid(Figure 1) as a source of Br+ for the efficient acetylation and formylation of a wide range of hydroxyl compounds under mild and solvent-free conditions(Scheme 1).
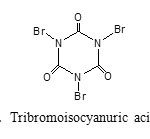 |
Figure 1: Tribromoisocyanuric acid (TBCA) Click here to View figure |
Initially, in order to find optimal conditions, we first examined acetylation reactions of 1 mmol of benzyl alcohol with different amounts of TBCA (0.05-0.2 mmol) and Ac2O (1-3 mmol) at room temperature under solvent-free conditions. It was observed that 0.2mmol of TBCA in 3mmol of Ac2O gave the best results and produced acetate in very short reaction time and in quantitative yields.
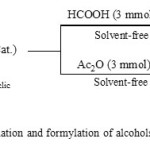 |
Scheme 1: Acetylation and formylation of alcohols and phenols Click here to View scheme |
The acetylation results under the optimized reaction conditions are shown in Table 1. The data in Table 1 clearly shows that primary and secondary alcohols and phenols were successfully converted to the corresponding acetate in short time and in almost quantitative yields. Primary and secondary benzylic alcohols with electron-releasing and electron-withdrawing groups were acetylated in the presence of TBCA and the corresponding acetate compounds were obtained in good to high yields (Table 1, entries 2-9). Also various phenols (Table 1, entries 10-15) and secondary cyclic alcohols (Table 1, entries 16-18) were successfully converted to the corresponding acetate in short time and in almost quantitative yields.
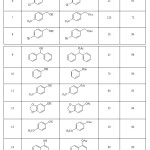 |
Table 1: Acetylation of alcohols and phenols with Ac2O in the presence of a catalytic amount of TBCA. |
aAll the products are known compounds and were identified by comparison of their physical and spectral data with those of authentic samples.
bIsolated pure product.
Subsequently, we discovered that formylation of a diverse range of alcohols can be carried out to obtain the corresponding formate with formic acid in the presence of the same catalyst in good to high yields under solvent-free conditions at room temperature (Table 2). As shown in Table 2, a series of structurally diverse alcohols including primary (benzylic, aliphatic) and secondary (benzylic, aliphatic, cyclic) were successfully converted to the corresponding acetate in short time and in almost quantitative yields.
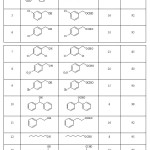 |
Table 2: Formylation of alcohols and phenols with formic acid in the presence of a catalytic amount of TBCA. Click here to View table |
The influence of electron-donating and electron-with drawing substituents at aromatic ring of alcohols is so notable. The alcohols with the electron- donating substituents on the aromatic ring (Table 1, entries 2-4 and Table 2, entries 2,3) increase the reaction rate but the alcohols with the electron-withdrawing substituents on the aromatic ring (Table 1, entries 5-8 and Table 2, entries 5-9) decrease the reaction rate in the both acetylation and formylation reactions.
References
- Greene, T. W., Protective Groups in Organic Synthesis, John Wiley & Sons, New York, (1981)
- Kocienski, P. J., Protecting Groups, Thieme, Stuttgart, Germany, (1994)
- Jadhav, V. H.; Kumar, K. S. A.; Chaudhari, V. D.; Dhavale, D. D. Synth. Commun. 2007, 37, 1363-1370
- Sreedhar, B.; Arundhathi, R.; Reddy, M. A.; Parthasarathy, G. Appl. Clay. Sci. 2009, 43 425-434
- Nowrouzi, N.; Mehranpour, A. M.; Ameri Rad, J. Tetrahedron. 2010, 66, 9596-9601
- Grasa, G. A.; Singh, R.; Nolan, S.P. Synthesis, 2004, 971-985
- Parmar, A.; Kaur, J.; Goyal, R.; Kumar, B.; Kumar, H. Synth. Commun. 1998, 28, 2821-2826
- Jomy, K.J.; Suman, L.J.; Bir, S. J. Mol. Catal. A: Chem. 2007, 267, 108-
- Kadam, S. T.; Kim, S. S. Synthesis, 2008, 267-268
- Mohammadpoor-Baltork, I.; Aliyan, H.; Khosropour, A. R. Tetrahedron, 2001, 57, 5851-5854
- Mirkhani, V.; Tangestaninejad, S.; Moghadam, M.; Yadollahi, B.; Alipanah, L. Monatsh. Chem. 2004, 135, 1257-1263
- Chakraborti, A.K.; Gulhane, R. Tetrahedron Lett. 2003, 44, 6749-6753.
- Dalpozzo, R.; Nino, A.D.; Maiuolo, L.; Procopiou, A.; Nardi, M.; Bartoli, G.; Romeo, R. Tetrahedron Lett. 2003, 44, 5621-5624
- Cope A. C. and Herrich E.C., Organic Synthesis Collective, vol. IV.; John Wiley. New York, (1963)
- Breton, G. W.; Kurtz, M.J.; Kurtz, S.L. Tetrahedron Lett. 1997, 38, 3825-3828
- Ishihara, K.; Kubota, M.; Kurihara, H.; Yamamoto, H. J. Org. Chem. 1996, 61, 4560-4567
- Procopiou, P. A.; Baugh, S. P. D.; Flack, S.S.; Inglis, A.G.G. Chem. Commun. 1996, 2625-2626
- Hagiwara, H.; Morohashi, K.; Sakai, H.; Suzuki, T.; Ando, M. Tetrahedron. 1998, 54, 5845-5852
- Orita, A.; Tanahashi, C.; Kakuda, A.; Otera, J. Angew. Chem. Int. Ed. 2000, 39, 2877-2869
- Iranpoor, N.; Firouzabadi, H.; Jamalian, A. Tetrahedron Lett. 2005, 46, 7963-7966
- Hill, D. R.; Hsiao, C. N.; Kurukulasuriya, R.; Wittenberger, S. J. Org. Lett. 2002, 4, 111-113
- Mirkhani, V.; Tangestaninejad, S.; Moghadam, M.; Yadollahi, B.; Alipanah, L. Monatsh. Chem. 2004, 135, 1257-1263
- Khazaei, A.; Zolfigol, M. A.; Rostami, A.; Ghorbani-Choghamarani, A. Catal. Commun. 2007, 8, 543-547
- Khazaei, A.; Rostami, A.; Mahboubifar, M. Catal. Commun. 2007, 8, 383-388
- Khazaei, A.; Rostami, A.; Raiatzadeh, A.; Mahboubifar, M. Can. J. Chem. 2007, 85, 336-340
- Khazaei, A.; Rostami, A.; Tanbakouchian, Z.; Zinati, Z. Catal.Commun. 2006, 7, 214-217

This work is licensed under a Creative Commons Attribution 4.0 International License.









