Tetrabutylammonium Bromide: An Efficient Catalyst for the Synthesis of Xanthenediones under Solvent-free Conditions
Ali Ezabadi*, Ramo Nazarian and Mina Gholami
Department of Chemistry, Faculty of Sciences, Central Tehran Branch, IslamicAzad University, SanatSquare, Iran Corresponding author E-mail: aliezabadi@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310334
Article Received on :
Article Accepted on :
Article Published : 22 Jul 2015
TBAB was found to be an effective catalyst for the synthesis of xanthenediones in good to excellent yields under solvent-free conditions. This method has many advantages such as avoiding the use of harmful solvents and catalysts, highyields and simple work-up.
KEYWORDS:Xanthenediones; Tetrabuty ammonium bromide; Solvent free conditions; Aromatic aldehydes; 5;5-Dimethyl.1;3-cyclohexanedion
Download this article as:| Copy the following to cite this article: Ezabadi A, Nazarian R, Gholami M. Tetrabutylammonium Bromide: An Efficient Catalyst for the Synthesis of Xanthenediones under Solvent-free Conditions. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Ezabadi A, Nazarian R, Gholami M. Tetrabutylammonium Bromide: An Efficient Catalyst for the Synthesis of Xanthenediones under Solvent-free Conditions. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=9868 |
Introduction
Xanthene derivatives especially xanthenediones have been attracting great interest because of their importance in synthetic organic chemistry. Many products that contain the subunit of xanthene exhibit use fuland diverse biological activities such as analgesic,1antiviral,2antibacterial,3 and anti-inflammatory activities.4Some of them have been used as antagonist for paralyzing the action of zoxazolamine,5 and in photodynamictherapy.6 Moreover, they can be use as dyes7 and are used extensivelyin laser technology8and pH sensitive fluorescent materials for visualization of biomolecules.9Many synthetic procedures for preparing xanthenediones have been reported by the condensation of aromatic aldehydes and 5,5-dimethyl-1,3-cyclohexanedione in the presence of alumina-sulfuric acid,10Fe3O4 nanoparticles,11 Fe3+-montmorillonite,12ZrOCl2.8H2O,13succinimide-N-sulfonic acid,14[Et3NH][HSO4],15 PMA-SiO2 ,16silica sulfuric acid,17nano Fe3O4@SiO2-SO3H,18DSIMHS,19 and zinc oxide nanoparticles.20
Although most of these methods offer distinctadvantages, they suffer from certain drawbacks such as high cost, unsatisfactory yields, the use of volatile organic solvents, stoichiometric amount of catalyst, and also environmentally toxic catalyst. Therefore, the search for green and readily available catalyst is still being actively pursed.
In recent years, tetrabutylammonium bromide (TBAB) has immerged as an extremely useful homogeneous catalyst invariousorganic transformations,21 including conjugate addition of thiols to electron deficient alkenes,22transthioacetalisation of acetals,23trimethylsilylation of alcohols,24synthesis of aryl-14H-dibenzo[a, j]xanthenes,25synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives,26synthesis of quinazolin-4(3H)-ones,27synthesis of tetrahydrobenzo[b]pyran derivatives,28and synthesis of optically active polyamides.29TBAB is an inexpensive readily available ionic liquid with inherent properties like environmental compatibility, greater selectivity, operational simplicity, non-corrosive nature and ease of reusability. Herein,we wish to report a simple and efficient method for the synthesis of xanthenediones from aromatic aldehydes, 5,5-dimethyl-1, 3-cyclohexane-dione and TBAB as acatalyst under solvent-free conditions(Scheme 1).
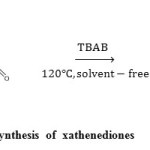 |
Scheme1: TBAB catalyzed synthesis of xathenediones Click here to View scheme |
Experimental
Material and Methods
All chemical compounds have purchased from Fluka, Romill and Merck companies and used without further purifications. The progress of the reaction was monitored by thin layer chromatography (TLC). Melting points were determined using Buchi B-540 melting point apparatus. FT-IR were recorded by JACSO FT-IR 410 spectrophotometer with KBr plates and 1H NMR spectra were recorded by Bruker DRX-500 MHz in CDCl3. Chemical shifts were expressed in δppm.
General procedure for the synthesis of xanthenediones under solvent-free conditions
A mixture of aromatic aldehydes (1 mmol), 5,5-dimethyl-1, 3-cyclohexanedione (2.2 mmol) and TBAT(40 mol%) under solvent-free conditions was heated at 120 °C and stirred for the required time (Table 2). After completion of the reaction, monitored by TLC, the reaction mixture was allowed to cool to room temperature. The crude product was recrystallized from hot ethanol to afford pure product.
Spectral data
3,3,6,6-Tetramethyl-9-(phenyl)-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3a): 1H NMR (CDCl3, 500 MHz) δ: 1.00 (s, 6H), 1.14 (s, 6H), 2.16-2.26 (q, 4H), 2.47 (s, 4H), 4.76 (s, 1H), 7.09-7.30 (m, 4H). IR (KBr) cm-1: 2954, 1661, 1364, 1198.
3,3,6,6-Tetramethyl-9-(4-chlorophenyl)-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3b): 1H NMR (CDCl3, 500 MHz) δ: 0.98 (s, 6H), 1.09 (s, 6H) 2.14-2.24 (q, 4H), 2.45 (s, 4H), 4.7 (s, 1H), 7.16-7.25 (m, 4H). IR (KBr) cm-1: 2956, 2877, 1662, 1364, 1197, 845.
3,3,6,6-Tetramethyl-9-(3-chlorophenyl)-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3c): 1H NMR (CDCl3, 500 MHz) δ: 0.99 (s, 6H), 1.09 (s, 6H) 2.15-2.24 (q, 4H), 2.46 (s, 4H), 4.71 (s, 1H), 7.06-7.25 (m, 4H). IR (KBr) cm-1: 2958, 2877, 1670, 1361, 1192, 1139.
3,3,6,6-Tetramethyl-9-(2-chlorophenyl)-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3d): 1H NMR (CDCl3, 500 MHz) δ: 1.00 (s, 6H), 1.09 (s, 6H) 2.13-2.23 (q, 4H), 2.43 (s, 4H), 4.98 (s, 1H), 7.04-7.41 (m, 4H). IR (KBr) cm-1: 2961, 1665, 1526, 1353, 1202.
3,3,6,6-Tetramethyl-9-(4-bromophenyl) -1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3e): 1H NMR (CDCl3, 500 MHz) δ: 0.97 (s, 6H), 1.09 (s, 6H) 2.01-2.21 (q, 4H), 2.45 (s, 4H), 4.68 (s, 1H), 7.15-7.32 (m, 4H). IR (KBr) cm-1: 2955, 1662, 1363, 1196.
3,3,6,6-Tetramethyl-9-(3-bromophenyl) -1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3f): 1H NMR (CDCl3, 500 MHz) δ: 1.00 (s, 6H), 1.1 (s, 6H) 2.16-2.26 (q, 4H), 2.46 (s, 4H), 4.7 (s, 1H), 7.08-7.35 (m, 4H), 8.07-8.09 (d, 2H) IR (KBr) cm-1: 2953, 1668, 1362, 1201.
3,3,6,6-Tetramethyl-9-(2-bromophenyl) -1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3g): 1H NMR (CDCl3, 500 MHz) δ: 1.01 (s, 6H), 1.09 (s, 6H) 2.13-2.22 (q, 4H), 2.44 (s, 4H), 5.01 (s, 1H), 6.94-7.44 (m, 4H). IR (KBr) cm-1: 2954, 1668, 1359, 1202, 1014, 742.
3, 3, 6, 6-Tetramethyl-9-(4-nitro-Phenyl)-1, 8- dioxo-1,2,3,4,5,6,7,8-octahydroxanthene (3h): 1H NMR (CDCl3, 500 MHz) δ: 0.98 (s, 6H), 1.11 (s, 6H) 2.14-2.26 (q, 4H), 2.44 (s, 4H), 4.81 (s, 1H), 7.46-7.48 (d, 2H), 8.07-8.09 (d, 2H). IR (KBr) cm-1: 2957, 1660, 1518, 1353, 1201.
3, 3, 6, 6-Tetramethyl-9-(3-nitro-Phenyl)-1, 8- dioxo-1,2,3,4,5,6,7,8-octahydroxanthene (3i):1H NMR (CDCl3, 500 MHz) δ: 0.99 (s, 6H), 1.11 (s, 6H) 2.01-2.26 (q, 4H), 2.50 (s, 4H), 4.83 (s, 1H), 7.25-8.00 (m, 4H). IR (KBr) cm-1: 2960, 1664, 1526, 1353, 1200.
3, 3, 6, 6-Tetramethyl-9-(2-nitro-Phenyl)-1, 8- dioxo-1,2,3,4,5,6,7,8-octahydroxanthene (3j):1H NMR (CDCl3, 500 MHz) δ: 0.99 (s, 6H), 1.08 (s, 6H) 2.13-2.23 (q, 4H), 2.45 (s, 4H), 5.5 (s, 1H), 7.21-7.75 (m, 4H). IR (KBr) cm-1: 2957, 1660, 1518, 1353, 1201.
3,3,6,6-Tetramethyl-9-(3-methoxy phenyl )-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3k): 1H NMR (CDCl3, 500 MHz) δ: 0.99 (s, 6H), 1.07 (s, 6H) 2.11-2.26 (q, 4H), 2.44-2.46 (q, 4H), 3.71 ( s, 3H), 4.81 (s, 1H), 6.69-7.35 (m, 4H). IR (KBr) cm-1: 2956, 1664, 1366, 1199.
3,3,6,6-Tetramethyl-9-(2-methoxy phenyl )-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3l): 1H NMR (CDCl3, 500 MHz) δ: 0.93 (s, 6H), 1.07 (s, 6H) 2.09-2.21 (q, 4H), 2.44-2.46 (q, 4H), 3.76 ( s, 3H), 4.84 (s, 1H), 6.73-7.41 (m, 4H). IR (KBr) cm-1: 2951, 1666, 1361, 1197, 754.
3,3,6,6-Tetramethyl-9-(4-fluoro phenyl)-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3m): 1H NMR (CDCl3, 500 MHz) δ: 0.98 (s, 6H), 1.09 (s, 6H) 2.14-2.24 (q, 4H), 2.45 (s, 4H), 4.71 (s, 1H), 6.86-7.25 (m, 4H). IR (KBr) cm-1: 2958, 1660, 1527, 1365, 1197, 845.
3,3,6,6-Tetramethyl-9-(4-hydroxy phenyl)-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3n): 1H NMR (CDCl3, 500 MHz) δ: 0.98 (s, 6H), 1.08 (s, 6H) 2.14-2.24 (q, 4H), 2.44 (s, 4H), 4.66 (s, 1H), 6.58-7.25 (m, 4H). IR (KBr) cm-1: 3358, 2960, 2868, 1660, 1363, 1200.
3,3,6,6-Tetramethyl-9-(2-thienyl)-1,8-dioxo- 1,2,3,4,5,6,7,8-octahydroxanthene (3o): 1H NMR (CDCl3, 500 MHz) δ: 1.99 (s, 6H), 1.02 (s, 6H) 2.25 (s, 4H), 2.44 (s, 4H), 5.14 (s, 1H), 6.81-7.25 (m, 3H). IR (KBr) cm-1: 2956, 2875, 1665, 1363, 1199.
Results and Discussion
During initial exploratory reaction, condensation of benzaldehyde and 5,5-dimethyl-1, 3-cyclohexanedione were taken as a model reaction to establish the feasibility of our strategy and to optimize reaction conditions, the results of which are summarized in Table 1. As expected, the catalytic system is influenced by various reaction parameters such as amounts of the catalyst employed (entries 1-4), effect of temperature(entries 4,5), amount of 5,5-dimethyl-1, 3-cyclohexanediones (entries 6) and solvent system(entries 7-11). Through thorough investigation, the best result in 92% yield was obtained by carrying out the reaction with 1:2.2 mol ratios of benzaldehyde and 5,5-dimethyl-1, 3-cyclohexanedione at 120°C and the dosage of 40 mol% catalyst for 8h under solvent-free conditions.
Encouraged by the remarkable results obtained with the above reaction conditions and in order to show the generality and scope of this new protocol,we performed the reaction with a variety of aromatic aldehydes with different substituents. The results are shown in Table 2. From the results, we found that all aromatic aldehydes carrying either electron-donating or electron-withdrawing group substituents reacted efficiently to give excellent yields. The high yield was also obtained in the case of the heterocyclic aldehyde (Table 2, entry 16). The desired products were characterized by 1H NMR, infrared(IR), melting points and also, by comparison with authentic samples.
Table1: The reaction of benzaldehyde and 5.5-dimethyl-1,3-cyclohexanedione under different reaction conditionsa
|
Entry |
Solvent |
Amount of catalyst (mol%) |
Temperature (°c) |
Yield b |
|
1 |
None |
10 |
120 |
– |
|
2 |
None |
20 |
120 |
– |
|
3 |
None |
30 |
120 |
82 |
|
4 |
None |
40 |
120 |
85 |
|
5 |
None |
40 |
100 |
– |
|
6 |
None |
40 |
120 |
92c |
|
7 |
H2O |
40 |
Reflux |
– |
|
8 |
CHCl3 |
40 |
Reflux |
– |
|
9 |
CH2Cl2 |
40 |
Reflux |
– |
|
10 |
EtOH |
40 |
Reflux |
20 |
|
11 |
EtOAC |
40 |
Reflux |
50 |
aReaction conditions: benzaldehyde (1 mmol), 5,5-dimthyl-1,3-cyclohexanedione (2 mmol)
bIsolated yield
cReaction conditions: benzaldehyde (1 mmol), 5.5-dimethyl-1,3-cyclohexandione ( 2.2 mmol), 8h
Table2: The synthesized of xanthenediones catalyzed by TBAB
|
Entry |
Ar |
Product |
Time (h) |
Yield (%) |
M.P. (°C) |
|
|
Found Reportedref |
||||||
|
1 |
C6H5 |
3a |
8 |
92 |
202-204 |
205-20630 |
|
2 |
4-ClC6H4 |
3b |
5 |
96 |
228-230 |
231-23319 |
|
3 |
3-ClC6H4 |
3c |
6 |
97 |
180-182 |
179-18113 |
|
4 |
2-ClC6H4 |
3d |
8 |
92 |
226-228 |
227-22819 |
|
5 |
4-BrC6H4 |
3e |
4.5 |
94 |
238-239 |
240-24219 |
|
6 |
3-BrC6H4 |
3f |
8 |
97 |
188-190 |
189-19119 |
|
7 |
2-BrC6H4 |
3g |
8 |
90 |
226-228 |
226-22931 |
|
8 |
4-NO2C6H4 |
3h |
5 |
97 |
225-227 |
222-22419 |
|
9 |
3-NO2C6H4 |
3i |
9 |
97 |
167-169 |
171-17218 |
|
10 |
2-NO2C6H4 |
3j |
8 |
70 |
256-258 |
250-25618 |
|
11 |
3-MeOC6H4 |
3k |
10 |
96 |
164-166 |
161-16332 |
|
12 |
2-MeOC6H4 |
3l |
12 |
85 |
253-255 |
257-25833 |
|
13 |
4-FC6H4 |
3m |
10 |
95 |
223-225 |
221-22319 |
|
14 |
4-OHC6H4 |
3n |
18 |
90 |
243-245 |
245-24719 |
|
15 |
C4H3S |
3p |
15 |
90 |
158-161 |
163-16510 |
aIsolated yield
Conclusion
In conclusion, we have explained an efficient method for the synthesis of xanthenediones catalyzed by TBAB as an inexpensive and readily available ionic liquid.The methodology has the advantages of high yield, lack of organic solvent, and easy work up for separation of products.
Acknowledgment
The authors are thankful to the Islamic Azad University of Central Tehran Branch for the support of this project.
Refrences
- Hafez, H.N.; Hegab, M.I.; Ahmed-Farag, I.S.; El-Gazzar, A.B.A.Bioorg.Med.Chem.lett. 2008, 18, 4538-4543.
- Lambert, R.W.;Martin, J.A.; Merret, J.H.;Parkes, K.E.B.; Thomas, G.J. PCT Int. Appl. WO9706178 (1997) Chem. Abstr. 126, P212377y.
- Hideu, T.Tokkyo Koho JP 56005480 (1981) Chem. Abstr. 95, 80922b.
- Poupelin, J.P.; Saint-Ruf, G.; Fussard-Blanpin, O.; Narcisse, G.; Uchida-Ernouf, G. Eur. J. Med. Chem. 1978, 13, 67-71.
- (a) Saint-Ruf, G.; Hieu, H.T. Arzneim. Forsch.1975, 25, 66-68; (b) G. Saint-Ruf,Hieu, H.T.; Poupelin, J.P.Naturwissenschaften. 1975, 62, 584-585.
- Ion, R.M.;Planner, A.; Wiktorowicz, K.; Frackowiak.Acta Biochim. Pol. 1998, 45, 833-845.
- Bhowmik, B.B.; Ganguly, P.Spectrochim. Acta, Part A. 2005, 61, 1997-2003.
- Ahmad, M.; King, T.A.; Do-Kyeong, K.; Cha, B.H.; Lee, J.J. Phys. D: Appl. Phys. 2002, 35, 1473-1476.
- Knight, C.G.; Stephens, T.Biochem. J. 1989, 258, 683-687.
- Pramanik, A.; Bhar, S.Catal. Commun. 2012, 20, 17-24.
- Karami, B.; Hoseini, S.j.; Eskandri, K.; Ghasemi, A.; Nasrabadi, H. Sci. Technol. 2012, 2, 331-338.
- Song, G.;Wang, B.; Luo, H.; Yang, L.Catal. Commun. 2007, 8, 673-676.
- Mosaddegh, E.; Islami, M.R.;Hassankhani, A.Arabian J. Chem. 2012, 5, 77-80.
- Shirini, F; Ghaffari-Khaligh, N.Dyes Pigm. 2012, 95, 789-794.
- Zhou, Z.; Deng, X.J. Mol. Catal. A: Chem.2013, 367, 99-102.
- Srihari, P.; Mandal, S.S.;Reddy, J.S.S.; Srinivasa, R.; Yadav, J.S.Chin. Chem. Lett. 2008, 19, 771-774.
- Seyyedhamzeh, M.; Mizaei, P.; Bazgir, A. Dyes Pigm. 2008, 76, 836-839.
- Naeimi, H.;Sadat-Nazifi, Z.J. Nanopart. Res. 2013, 15, 2026-2032
- Shirini, F.; Yahyazadeh, A.; Mohammadi, K.Chin. Chem. Lett. 2014, 25, 341-347.
- Safaei-Ghomi, J.;Ghasemzadeh, M.A. Chin. Chem. Lett. 2012, 23, 1225-1229.
- (a) Smietana, M.;Mioskowski, C.Org. lett. 2001, 3, 1037-1039; (b) Amantini, C.;Fringuelli, F.;Vaccaro, L.J. Org. Chem. 2001, 66, 6734-6737; (C) Selvakumar, K.; Zapt, A.;Beller, M.Org. Lett. 2002, 4, 3031-3033.
- Ranu, B.C.;Dey, S.S.;Hajra, A. Tetrahedron. Lett. 2003, 59, 2417-2421.
- Ranu, B.C.;Das, A.;Samanta, S. J. Chem. Soc. Perkin Trans. 2002, 1, 1520-1522.
- Amantini, D.; Fringuelli, F.; Pizzo, F.; Vaccaro, L. J. Or;g. Chem. 2001, 66, 6734-6737.
- Kantevari,S.; Chary, M.V.; Das, A.P.R.; Srinivasu, V.N.V.; Lingaiah, N. Catal. Commun. 2008, 9, 1575-1578.
- Khurana, J. M.; Kumar, S. Tetrahedron. Lett. 2009, 50, 4125 – 4127.
- Davoodnia, A.; Allameh, S.; Fakhari, A.R.; Tavakoli-Hoseini, N. Chin. Chem. Lett. 2010, 21, 550-553.
- Gurumurth, S.; Sundari, V.; Valliappan, R. J. Chem. 2009, 6, 5466-5472.
- Mallakpour, S.; Taghavi, M. J. Appl. Polym. Sci. 2008,109, 3603-3612.
- Venkatesan, K.; Pujari, S.S. Lahoti, R.J.; Srinivasan, K.V. Ultrason. Sonochem. 2008, 15, 548-553.
- Zhan-Hui, Z.; Yu-Heng, L. Catal. Commun. 2008, 9, 1715-1719.
- Zare, A.; Moosavi-Zare, A.R.; Merajoddin, M.; Zolfigol, M.A.; Hekmat-Zadeh, T.; Hasaninejad, A.; Khazaei, A.; Mokhlesi, M.; Khakyzadeh, V.; Derakhshan-Panah, F.; Beyzavi, M.H.; Rostami, E.; Arghoon, A.; Roohandeh, R. J. Mol. Liq. 2012, 167, 69-77.
- Kidwai M.; Jain, A. Appl. Organomet. Chem. 2012, 26, 528-535.

This work is licensed under a Creative Commons Attribution 4.0 International License.









