Synthesis of Thioacridine Derivatives Using Lawesson’s Reagent
Palla Mahesh1,2*, B.Dilip Kumar1, B. Rama Devi2 and Y.L.N. Murthy1
1Department of Organic Chemistry, Andhra University, Visakhapatnam, India-530 003. 2Department of Chemistry, Jawaharlal Technological University, Kukatpally Hyderabad, India-500 085. Corresponding Author Email: mahi143vzm@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310347
Article Received on :
Article Accepted on :
Article Published : 31 Jul 2015
The synthesis of thioacridine derivatives (5a-j) have been achieved by the reaction of acridines (4a-j) with Lawesson’s reagent in toluene under refluxing conditions to yield products in high yields. The yields of the products are promising and the products are characterized by advanced spectroscopic studies.
KEYWORDS:Acridines; thioacridines; Lawesson’s reagent
Download this article as:| Copy the following to cite this article: Mahesh P, Kumar B. D, Devi B. R, Murthy Y. L. N. Synthesis of Thioacridine Derivatives Using Lawesson’s Reagent. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Mahesh P, Kumar B. D, Devi B. R, Murthy Y. L. N. Synthesis of Thioacridine Derivatives Using Lawesson’s Reagent. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10132 |
Introduction
Acridines represent an important class of nitrogen heterocycles having several significant properties such as pigment, dye properties, photochemical/physical properties, electrochemical properties, potent anti-malarial, anticancer and anti-fungal activity etc [1, 2]. Natural and synthetic acridines and their derivatives are effective DNA and RNA-binding compounds owing to their intercalation abilities as well as being a lipophilic carrier molecule [3, 4]. Synthesis of Acridinediones is a continuing focal point of current research because these moieties are active pharmaceutical ingredients (API’s) and also valuable reactive intermediates for both synthetic and medicinal chemists [5].
Literature survey reveals that various methods [6] have been reported for preparation of acridinediones and substituted acridinediones. The reported method for the synthesis of 9-aryl-3, 3, 6, 6-tetramethylhexahydroacridine-1, 8-diones involves the reaction of two molecules of dimedone (5,5-dimethyl-1,3-cyclohexane) with various aromatic aldehydes and ammonium acetate by using different Lewis acid catalysts [7]. However many of these catalysts undergo disadvantages such as long reaction time, high catalyst loading, use of solvents and deactivation of catalyst on repeated use. Hence there is a need to develop an environmentally benign protocol for the synthesis of acridinediones.
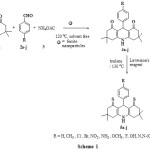 |
Scheme1: Synthesis of 9 – aryl – 3, 3, 6, 6-tetramethyl hexahydro thioacridine-1,8-diones (5a-j) Click here to View scheme |
In this study, we report a simple, efficient and one-pot reaction of dimedone (1), aldehydes (2a-j) and ammonium acetate (3) using nano ferrite at 120 oC for the synthesis of acridinediones (4a-j). These acridinediones (4a-j) on reaction with Lawesson’s reagent using toluene as solvent at 130 oC yields thioacridines (5a-j) which was presented in Scheme-1.
Materials and Methods
Materials
1,3-Diketones and Aromatic aldehydes were obtained from Aldrich chemicals. Melting points were determined on a Buchi 504 apparatus and are uncorrected. IR spectra were recorded in KBr pellets on a Nicolet (Impact 410) FT-IR spectrophotometer. 1H NMR and 13C NMR spectra were recorded on a Varian Mercury Plus 400MHz NMR spectrophotometer using tetramethylsilane (TMS) as an internal standard. Coupling constants are expressed in hertz. The progress of the reaction was monitored by thin layer chromatography (TLC) that runs on silica gel G (Merck).
General procedure for the synthesis of 9-aryl substituted-3,3,6,6-tetramethylhexahydro acridine-1,8-diones (4a-j):
A mixture of 5, 5-dimethyl-1, 3-cyclohexanedione (2 eq) (1), substituted aromatic aldehydes (1 eq) (2), Ammonium acetate (3) and Fe2O3 nano particles (15 mol %) were stirred at 120 oC in an oil bath for the prescribed time. The completion of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature to attain solid. The obtained solid was dissolved in methanol and the catalyst was recovered by magnetization. The crude products were further purified by recrystallization from ethanol. All the synthesized products were characterized by IR, NMR and Mass spectroscopic data and their melting points were compared with authentic samples.
General procedure for the synthesis of 9-aryl substituted-3,3,6,6-tetramethylhexahydro thioacridine-1,8-diones (5a-j):
A mixture of acridine (4a-j) (1 eq), Lawesson’s reagent (2 eq) in presence of toluene were refluxed at 130 oC in an oil bath. The completion of the reaction was monitored by TLC. After completion of the reaction, toulene was evaporated under reduced pressure to obtain solid. The solid was dissolved in EtOAc and washed with excess of water. The organic layer was collected, dried with Anhy. Na2SO4 and EtOAc was removed using rota vapour to obtain crude products. The crude products were purified by recrystallization from ethanol. All the synthesized product were characterized by IR,NMR and Mass spectroscopic data.The Reaction times percentage of yield and were presented in table-1 the spectral data of some of the synthesized compounds are given below.
3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dithione (5a) : IR (KBr) (νmax,cm-1): 2985,1680,1370,1226 cm-1. 1H NMR (400MHz, DMSO): δ 9.24(s, 1H), δ7.15-7.09 (m, 5H, Ar H), 4.64 (s, 1H, CH), 2.46 (s, 4H, 2CH2), 2.19 (q, J=16.5 Hz,4H, 2CH2), 1.10 (s, 6H, 2CH3), 0.98 (s, 6H, 2CH3). 13C NMR (75 MHz, DMSO-d6): δ 196.2, 162.3, 143.8, 128.4, 127.6, 115.2, 50.6, 40.7, 32.0, 31.6,29.3, 27.2. MS (m/e): 382 (M+).
9-(4-bromophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dithione (5d): IR (KBr) (νmax,cm-1):2930, 1624, 1286;1H NMR (75 MHz, DMSO-d6): δ9.35(s, 1H), δ H 6.47 (1H, s, CH),7.25e8.34 (4H, m, Arom.);13C NMR (DMSO-d6): δC 37.44,116.55, 118.00, 120.22, 122.35, 124.65, 126.86, 128.85,129.23, 129.57, 131.12, 131.78, 131.24, 143.98, 148.65; MS(m/e, %)460[M+]. .
Table1: Synthesis of Thioacridin derivavives
|
S.No |
R |
Time(min) |
Yield(%)a |
|
5a |
H |
10 |
90% |
|
5b |
-CH3 |
15 |
91% |
|
5c |
-Cl |
13 |
85% |
|
5d |
-Br |
15 |
80% |
|
5e |
-NO2 |
15 |
90% |
|
5f |
-NH2 |
16 |
88% |
|
5g |
-OCH3 |
15 |
90% |
|
5h |
-F |
14 |
91% |
|
5i |
-OH |
12 |
92% |
|
5j |
-N,N(CH3)2 |
14 |
85% |
a Isolated yield after column chromatography.
Results and Discussion
Initially, a blank reaction was performed using benzaldehyde (2a), dimedone (1) and ammoniumacetate (3) (mole rate 1:2:1) at 120 oC in the absence of nano ferrite to establish the efficacy of the catalyst and the results showed that desired product was not formed even after 12 hours of heating. Then focus was diverted to optimize amount of catalyst and solvent effect. In order to evaluate the most appropriate catalyst percentage, a model reaction using benzaldehyde (2a), dimedone (1) and ammoniumacetate (3) to form acridines.
A mixture of acridines (4a-j) and Lawesson’s reagent in presence of toluene were refluxed at 130oC in an oil bath for 15 min to form thioacridines (5a-j). The completion of the reaction was monitored by TLC and th products are purified by coloumn chromatography and are recrystallisd from ethanol.
The thionation of carbonyl oxygen has been achieved by different reagents like phosphorous pentasulfide (P4S10) bis(trimethyl sillyl) sulphide-cobalt chloride ammonium phosphorodithioate as an efficient thionation reagent, PSCl3/H2O/Et3, etrachlorosilane (TCS) and sodium sulfide. But all these catalysts need drastic conditions and high reaction times. But we found from literature that Lawesson’s reagent is one of the best thionating agents. Here in this reaction the thionation was achieved by simple process and the reaction proceeds at a higher rate with good yields. At the outset, the role solvent is studied to identify the best solvent system to get more yields in less time. Different protic and aprotic solvents are tested and the results are presented in table 3.From the tables it is observed that the reaction proceeds at a higher rate with more yields in the presence of Toluene (Table 2, entry 4).
After attaining the optimized conditions, a model reaction was performed with a simple acridinone (4a) and after completion of the reaction the product (5a) was purified and recrystallized. From the IR spectroscopy of compound 5a, it was observed that the characteristic –C=S stretching vibrations are observed at 1226 cm-1 which confirms the formation of thio compound and by HRMS (M+ at 382) study we confirmed the formation of thioacridinone.
Table2: Solvent Effect.
|
Entry |
Solvent |
Time(h) |
Yield(%)a |
|
1 |
Acetonitrile |
12 |
10 |
|
2 |
THF |
12 |
15 |
|
3 |
Ethanol |
12 |
5 |
|
4 |
Toluene |
25(min) |
90 |
|
5 |
1,4-Dioxane |
12 |
13 |
|
6 |
Methanol |
12 |
15 |
|
7 |
Wather |
24 |
0 |
a Isolated yield after column chromatography.
Conclusions
In conclusion, a convenient and highly efficient method for the synthesis of thioacridines in presence of Lawesson’s reagent was reported. The attractive features of this synthetic protocol are easy work up procedure and operational simplicity. Furthermore, products were isolated in good to excellent yields.
Acknowledgements
The authors are thankful to Defence Research and Development Organization (DRDO), New Delhi, India for providing financial assistance. The authors are also grateful to the Committee On Strengthening Infrastructure for Science & Technology (COSIST) Labs, Andhra University, India for providing spectral data.
References
- Albert, A., The Acridines; St.Martin’s Press; New York, 1966, 403-504.
- Guetzoyan, L., Yu, X.M.; F. Ramiandrasoa; Peth, S; Rogier, C; Pradines, B; Cresteil; Bioorg Med. Chem. 2009, 17, 8032-8039.
- Topcu, Z; Clin. Pharm. Ther. 2001, 26, 405-416.
- Van Mouwerik, T.J.; Caines, P.M.; Ballentine, R, Drug Intell. Clin. Pharm. 1987, 21, 330-334.
- Heravi; M.M; Bakhtiari, K; Zadsirjan, V.; Bamoharram, V, Bioorg Med. Chem. 2007, 17, 4262-4265
- Smolders, R.R.; Waefelaer, A; Coomans, R; Francart, R; Hanuise, J; Bull. Soc. Chim. Belg. 1982, 91, 33-42.
- Kidwai, M; Bhatnagar, D. Tet. Lett. 2010, 51, 2700-2703
- Velu, R; Malar, E.J.P.; Ramakrishna, V.T., Ramamurthy, P; Tet. Lett, 2010, 51, 5680-5685.

This work is licensed under a Creative Commons Attribution 4.0 International License.









