Study of the profile concentration of a chemical through a sandwich packaging
Rachid Atmani1*, M’hammed El Kouali1, Mohammed Talbi1, Abdlhak El Brouzi2, Fatima Ouzidan1
1Research Laboratory of Analytical Chemistry and Physical Chemistry of Materials, Sciences Faculty Ben M'sik, University Hassan II - Casablanca, Morocco
2Research Laboratory of Physical Chemistry and Bioorganic. Faculty of sciences and Techniques Mohammedia. University Hassan II-Casablanca.
Correspondence Author Emails: atmanirachid12@gmail.comDOI : http://dx.doi.org/10.13005/ojc/310344
Article Received on :
Article Accepted on :
Article Published : 20 Aug 2015
The process of chemical transfer is studied through a sandwich packaging, we have been determined the profile of concentration of a chemical through a sandwich packaging in order to understand this phenomenon. The method used for determining the profile of concentration of the chemical in the polymer, based on numerical analysis, given in two parts:
- The first part is done by putting in contact two polymer sheets, one containing the chemical while the other is free from this chemical. The diffusivity when the chemical leave the first sheet is same when this chemical enters inside the other sheet.
- The second part is done by putting the sheet of the polymer containing the chemical in contact with two virgin sheets. The diffusivity when the chemical leave the first sheet is same when this chemical enters inside two other sheets.
Sandwich packaging; Plastic; Modeling; Finite D.M; Diffusion
Download this article as:| Copy the following to cite this article: Atmani R, El Kouali M, Talbi M, El Brouzi A, Ouzidan F. Study of the profile concentration of a chemical through a sandwich packaging. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Atmani R, El Kouali M, Talbi M, El Brouzi A, Ouzidan F. Study of the profile concentration of a chemical through a sandwich packaging. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10308 |
Introduction
Polymer Materials take a good place in the packaging field [4], so their contact with some product two types of matter transfer occur [4]. First type when the liquid entering the polymer while the second one same additive or chemicals leave the polymer. Both these transfers are governed by transient diffusion.
The purpose of this study is applied a methods which consists of developing the concentration profile developed through the polymer at various time, this method using to determine the kinetics of change in weight.
Both cases were investigated:
- When two sheets are in contact, one containing the chemical while the other is free from this chemical
- Making two sheets in contact with another sheet containing the chemical.
The second objective of this study is to build a numerical model able to resolve the problem.
Materials and Methods
Assumptions
Some assumptions are made so as to simplify this phenomenon [4] [5].
- In the case of the two sheet system, one sheet is free from chemical, while other contains a chemical.
- In the case of the three sheet system, the sheet containing the chemical located between two virgin sheets.
- The concentration initially is uniform
- The transfer is perpendicular to the face of sheet
- The matter transfer is governed by transient diffusion
No evaporation of the chemical.
Mathematical treatment
The general equation of one-directional diffusion with constant diffusivity is [3]:
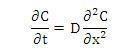
With the following initial conditions:
t = 0 Sheet with chemical C = Cin
Sheet free from chemical C = 0
And t >0 external surfaces ðC/ðx = 0
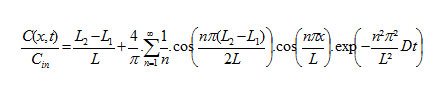
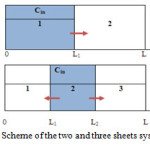 |
Figure 1: Scheme of the two and three sheets systems Click here to View figure |
At the interface between the two polymers sheets, we have [2]:
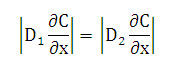
That is mean the rate of liquid transfer is the same on each face of the interface.
The partition factor is the ratio of the concentrations of liquid on each face of the interface:
K= C2/C1 At the interface
Analytical Solutions
Firstly, it should be noted that the diffusivity is the same in two sheets.
Case of two Sheet Systems
The profile of concentration of chemical developed through the two sheet systems is given by [3]:
With:
- L1 is the thickness of the diaper where the chemical is initially placed.
- L is the total thickness of the two sheets.
- D is the diffusivity of the chemical in the polymer of the two sheets.
Case of three Sheet Systems
The profile of concentration of chemical developed with three sheets is given by [3]:
With:
- (L2-L1) is the thickness of the sheet where the chemical is initially placed.
- L is the total thickness of the two sheets.
- L1 and (L-L2) are the thickness of each virgin polymer sheet.
- D is the diffusivity of the chemical in the polymer of the three sheets.
Numerical solution
For all cases, whether an analytical solution exists or not, the numerical treatment is feasible.
We used a Finite difference method (FDM) solution in order to resolve the problem numerically; the principle of this method is to let the range in x be divided into N equal intervals of ∆x and time into M equal intervals of∆t. A random point in space and time (x, t) can be represented, by (i∆x, j∆t), where i and j are integers (i = 1,2,…,N+1 and j = 0,1,…,M).When the profile of chemical concentration is not initially uniform in the source sheet [2], the numerical treatment is the only feasible method [1, 2].
Experimental Procedure
We used a scientific software in order to solve the equations for each treatment.
Results and Discussion
The results are presented in terms of profiles of concentration developed for two and three sheet systems (fig.1) and kinetics diffusion for each system.
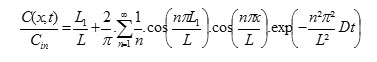
For two sheet systems, the chemical is located initially in the sheet on the left. Dimensionless numbers are used so as to make the results general.
The coordinates for this contact are: the concentration C at position x and time t as a fraction of the initial concentration Cin; the position x as a fraction of the total thickness L of the sheets system. Dimensionless time is D∆t/L2. where L is the total thickness of the system.
The results for each system are presented in two following tables:
 |
Figure 2: Evolution of mass in two sheets with simulation time |
 |
Figure 3: Profiles of concentration developed through the two sheets system at different times, with the same thickness, (L1/L=2) same diffusivity (D1/D2 =1) and K =1. Click here to View figure |
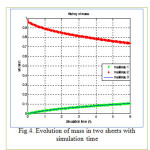 |
Figure 4: Evolution of mass in two sheets with simulation time Click here to View figure |
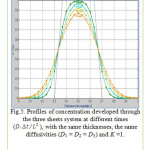 |
Figure 5: Profiles of concentration developed through the three sheets system at different times (D.Δt/L2), with the same thicknesses, the same diffusivities (D1 = D2 = D3) and K =1. Click here to View figure |
Conclusion
In this article, we studied theoretically two and three sheet systems for determining the profiles of concentration of a chemical developed in these sheets when this chemical was initially placed in one sheet. From the profiles developed we can conclude:
Firstly, these profiles give further information about the nature of the process of the chemical transfer.
Secondly, the diffusivity in a sheet containing the chemical depend the diffusion coefficient in the other sheet virgin.
References
- J. Crank, the Mathematics of Diffusion, Clarendon Press, Oxford, 1975.
- J.M. Vergnaud, Liquid Transfer Processes in Polymeric Materials, Prentice Hall Publishers, Englewood Cliffs, NJ,USA, 1991.
- J. M. VERGNAUD, «Liquid Transparent Process in Polymeric Materials. Modelling and Industrial Applications», Prentice Hall, Englewood Cliffs, NJ, 1991.
- Rachid ATMANI, 2014, «Diffusion of Heptane in Polyethylene Vinyl Acetate: Modelisation and Experimentation», IOSR Journal of Applied Chemistry (IOSR-JAC), 2014 .,7, (6.). 82-86.
- Rachid ATMANI, , «Ethanol Diffusion in Polyethylene Vinyl Acetate: Modelling and Experimentation», Global Journal of Science Frontier Research Chemistry, 2013., 13 (8).

This work is licensed under a Creative Commons Attribution 4.0 International License.









