Magnetic and Spectral Study of Some Mixed Ligand Complexes
Bishwanath Kumar1, Rajeev Kumar2 and Birendra Kumar1*
1Department of chemistry, Jagjiwan College, Gaya. chemistry, S D College, Paraiya 2Department of chemistry, Maha Bodhi College, Belaganj. Correspondence Author Emails: birendra5556@yahoo.in
DOI : http://dx.doi.org/10.13005/ojc/310366
Article Received on :
Article Accepted on :
Article Published : 21 Aug 2015
Complexes of Fe(II), Co(II) and Ni(II) metal ions have been prepared with 4-(p-hydroxyl benzalidimino)-3,5-dimercapto-1,2,4-triazole (LH) as primary ligand while pyridine has been used as secondary ligand. The analytical data suggest 1:2 (M:L) stoichiometric composition of complexes . The electrical conductivity indicates their non- electrolytic nature. The magnetic and spectral character favour octahedral geometry of Fe(II), Co(II) and Ni(II) complexes.
KEYWORDS:Precursor; Heterochelates
Download this article as:| Copy the following to cite this article: Kumar B, Kumar R, Kumar B. Magnetic and Spectral Study of Some Mixed Ligand Complexes. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Kumar B, Kumar R, Kumar B. Magnetic and Spectral Study of Some Mixed Ligand Complexes. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10386 |
Introduction
The schiff’s bases of triazole derivative and their metal complexes possess pharmacological acivities.1-8 It is found that pharmacological activity of triazole derivative is enhanced ridiculously on coordination with metal ions.6 Present paper deals with some mixed ligand complexes of Fe(II), Co(II) and Ni(II) metal ions
Material and Methods
The organic reagent and solvent used in present investigation were procured from B.D.H. and Bengal chemicals. Metal salts of Iron (II), Nickel (II) and Cobalt (II) used were all of either Merck or of B.D.H., A.R Grade quality. The primary ligand, 4-(p-hydroxyl -benzalidimino)-3,5-dimercapto-1,2,4-triazole was prepared. For this at first monopyridinium salt of 4-amino-3,5 dimercapto-1,2,4-triazole was prepared by refluxing the mixture of thiocarbohydrazide and carbon disulphide in pyridine on an oil bath for 1 hr. From boiling solution a precipitate appeared and after cooling, pale yellow prismatic crystal was obtained. The precursor, 4-amino-3,5 dimercapto-1,2,4-triazole was prepared by heating the obtained pyridinium salt with HCl. This amine was used to synthesize the primary ligand, i.e., 4-(p-hydroxylbenzalidimino)-3,5-dimercapto-1,2,4-triazole, by refluxing the equimolor quantity of 4-amino-3,5 dimercapto-1,2,4-triazole and p-hydroxybenzaldehyde in ethanol for 1 hr. The reaction mixture was allowed to stand overnight at room temperature where by yellow crystal was obtained.
The alcoholic solution of 0.005 M metal chloride and the alcoholic solution of 0.01 M ligand were mixed together with 2 ml of α- picoline. The mixture was refluxed on water bath for one and half hour at low temperature. On cooling the solid separated out. The solid complex so obtained in each case was filtered, washed several times with hot water and finally with alcohol. It was dried in desiccator over anhydrous calcium chloride. The ligand and metal complexes were analyzed for C, H, N, S and metal. Molar conductance of metal complexes was determined. For this 10-3M solution in DMSO was used. Magnetic susceptibility and µeff of metal complexes were determined by Gouy’s balance method9.
Result and Discussion
The analytical data given in table: 1 show 1:2 (M:L) stoichiometric composition of metal complexes. Low values of molar conductance of all the complexes indicate their non-electrolytic nature. A weak band at 2560 cm-1 in IR spectrum of free ligand (L) is assigned to ν (SH) stretching vibration. Disappearance of this band in IR spectra of complexes indicates the deprotonation of mercapto group and coordination to metal ions through deprotonated mercapto sulphur10. A strong band appearing at 2850cm-1 in IR spectrum of free ligand may be assignable to H-bonded phenolic –OH group, which remains unaltered in the spectra of complexes indicating its non-participation in complexation.11A band at 1628 cm-1 in the IR spectrum of free ligand is assigned to azomethine group. Blue shift (15-24 cm-1) of this band in the IR spectra of metal complexes is indicative of coordination through azomethine nitrogen of ligand.12 Three bands at 2910 cm-1, at 3050 cm-1 and at 1456 cm-1 appearing in the IR spectra of metal complexes are assignable to νCH of methyl group aromatic, νCH and νC=N of α-picoline molecules respctively.13-15 A band at 755 cm-1 is appeared in the IR spectra of metal complexes. This may be due to coordinated α-picoline to metal ions in complexs.16-19 The non-ligand band at 315 cm-1and 265 cm-1 in spectrum of Ni- complex are assignable to ν (M-N) and ν (M-S) respectively. The non-ligand band at 320 cm-1and 270 cm-1 in spectrum of Co- complex are assignable to ν (M-N) and ν (M-S) respectively. The non-ligand band at 320 cm-1 and 280 cm-1 in spectrum of Fe- complex are assignable to ν (M-N) and ν (M-S) respectively. Study of IR spectra of metal complexes reveals that the ligand has been found to be coordinated though thiol sulphur and azomethine nitrogen, so the chelates may be called to be heterochelates.
Magnetic moment (µeff) value of Ni (II) complex is 2.80 B.M. which is in the better agreement with octahedral Ni(II) complexes.20-22 Magnetic moment (µeff) for Co (II) complex is determined and found to be 2.60 B.M. at room temperature . This µeff value is intermediate between those of low-spin octahedral or planar and high-spin octahedral Co (II) complexes. This anomalous value of magnetic moment for Co (II) complex may be attributed to spin state equilibrium between high spin and low spin species. Magnetic moment (µeff) for Fe(II) complex is determined and found to be 5.25 B.M. which is slightly greater than that of the value of spin free octahedral Fe(II) complexes. This may be attributed to appreciable contribution from triply degenerate ground state term, i.e., 5T2g. This value of µeff of Fe (II) complex is in good agreement with the reported values for spin free octahedral Fe (II) complexes.23-25
The electronic spectra of Ni(II) complex exhibits three bands at 11280cm-1, 16840cm-1 and 26190cm-1 which are assignable to the transition 3A2g(F)→3T2g(F)( ν1), 3A2g(F)→3T1g(F)( ν2) and 3A2g(F)→3T1g(P)( ν3) respective -ly. The various crystal field parameters of Ni (II) complex, viz, 10Dq, B and β were calculated. The value of 10Dq, B and β are found to be 11290cm-1, 616.4cm-1 and 0.59 respectively. The values of different crystal field parameters are in good agreement with reported values of octahedral Ni(II) complexes.26-29 The electronic spectra of Co(II) complex exhibits three bands at 9755cm-1, 17310cm-1 and 23515cm-1 which are assigned to the transition 4T1g(F)→4T2g(F)( ν1), 4T1g(F)→4A2g(F)( ν2) and 4T1g(F)→4T1g(P)( ν3) respectively. The various crystal field parameters of Co (II) complex, viz, 10Dq and B are calculated. The value of 10Dq and B are found to be 7555cm,-1 and 1266cm-1 respectively. The values of different crystal field parameters are in good agreement with reported values for octahedral Co(II) complexes.30-33 A broad band having shoulder at 11810cm-1 is appeared in electronic spectrum of Fe(II) complex. The spectral band is assigned to 5T2g→5Eg transition.Shoulder in electronic band is indicative of the distortion from octahedral symmetry. Thus Fe complex has distorted octahedral symmetry. 34-37
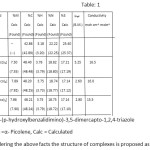 |
Table 1 Click here to View table |
LH = 4-(p-hydroxylbenzalidimino)-3,5-dimercapto-1,2,4-triazole
α-pico =α- Picolene, Calc = Calculated
Considering the above facts the structure of complexes is proposed as follow: –
M=Fe, Ni, Co
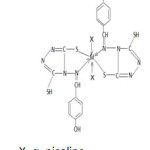 |
Figure 1 Click here to View figure |
Acknowledgement
The authors are thankful to then Principal, Gaya College, Gaya, Dr. S.K. Sharma for providing laboratory facilities. The authors take opportunity to gratefully acknowledge to Dr. Shivadhar Sharma. Professor and Head, University Deptt. of Chemistry, Magadh University, Bodh Gaya for his valuable suggestion and sincere advice.
References
- Chohan, Z.H.; Ruff, A.; Supuran, C.T. Metal Based Drug. 2001, 8, 287
- Piotr, P.; Adam, H.; Krystian, P.; Bogmil, B. and Franz, B. Curr. Org. Chem, 2009,13,124
- Ghosh , S.; Malik, S.; Jain, B.; Gupta, M. J. Indian Chem. Soc., 2012, 89, 471.
- Holm, R.H.; Connev, M. J. Prog. Inorg. Chem., 1971,41,253.
- Sacconi, L. Transition Metal Chem., 1968,4,199..
- Patil, S.; Bodiger, B.M.; Kudari ,S.N.; Kulkarni, V.R. Transition MetalChem., 1983,45,879
- Jagmohan, K.S.D.; Chadha, V.K.; and Pujari, H.K. Indian J. Chem., 1974, 12, 288.
- Shafranski, V.N.; Stralulat, A.A.; Dranka, I.V. Russ. J. Inorg. Chem., 1981,26,975.
- Figgiis, B.N.; Lewis,J. Modern Inorganic Chemistry, Interscience New York, (1960)
- Sharma Y.R., Elementary Organic Spectroscopy, S. Chand & Co. Pvt. Ltd. New Delhi, Revised Edition, (2013)
- Kumar, B.; Verma, P.K. J. Chemtracks, 2013,15(1), 249
- Dubey,R.K.; Mishra, S.K.; Mariya, A.; Mishra, A.K. J. Indian Chem. Soc., 2013,90,29,
- Singh, M.K.; Lasker, A.D.R.; Paul, B. J. Indian Chem. Soc. 2008, 85, 485.
- Chandra, S.; Jain, D.; Sarkar, A. J. Indian Chem. Soc. 2009, 86, 220
- Saha, N. Indian J Chem. 1981, 20A, 680
- Fraerzer ; Glowacky . J. Am. Chem. Soc. 1948, 70, 2575
- Yamada, H. Appl. Spectrosc.Rev. 1981, 17, 227
- Gill, N.S.; Kingdom,H.J. Aust. J Chem. 1966, 19,2197
- Liver, A.B.P.; Ramaswami, B.S. . Indian Canad.J Chem. 1973, 51, 1582
- Ray, P.; Sen, D. J. Indian Chem. Soc., 1948,25, 473
- Figgis and Narris, J. Chem. Soc. 1959, 855
- Singh, N.P.; Anand, K. L.; Singh, J. Asian J. Chem., 2011, 23, 4090
- Mallik , S.; Kumari, M.; Sharma, D.K. J. Indian Chem. Soc. 2010, 87, 539
- Dubey, R.K.; Mishra, C.K.; Dwivedi S.K.; Tripathi, U.N. J. Indian Chem. Soc. 2011, 88, 1605
- Prasad, R.N.; Sharma, P. J. Indian Chem. Soc. 2012, 89, 861
- R. Goalbedaghi, Asian J. Chem.2011, 23,4239
- Zabin, S.A. Asian J. Chem.2011, 23,4067.
- Prasad , R.N.; Sharma, P. J. Indian Chem. Soc.2012, 89,865.
- Bose, S.; Mallile, S.; Jain; B. Gupta, M. J. Indian Chem. Soc. 2012,89, 471.
- Singh, P.K.; Singh, S. J. Chemtracks, 2010, 12 (2), 303.
- Singh, A.K.; Singh, K.S.; Kumar, A.; Kumar, S.; Sharma, S. J. Chemtracks, 2010,12 (2), 503
- Kumar, A.; Khasa, A.; Sharma, P.G.; Rai, A. Asian J. Chem.2011, 23, 4494.
- Rai, B.K.; Kumar, B. Asian J. Chem. 2011, 23,4635.
- Soloshmok, V.A.; Veki, H.; Ellis, T.K.; Yasoda,T.; Ohtume, Y., Tetrahedron Lett. 2005, 46, 1107.
- Rajlakshmi, V.; Vijayraghavan, V.R.; Vargese, B.; Raghavan , A. J. Inorg. Chem. 2008, 47 A,5821.
- Damiel, V.P.; Pillai, T.P.C.; Kumari, B.S.; Joseyphus R.S.; Mohanan, D K. J. Indian Chem. Soc. 2011,88, 1639.
- Sathyamarayana , B.N., Electronic absorption spectroscopy and related technique. University Press Ltd. Hyderabad, (2011)

This work is licensed under a Creative Commons Attribution 4.0 International License.









