Determination of Cyproheptadine hydrochloride in Pure and Pharmaceutical forms : A Spectrophotometric study
Sayanna1, T. Veeraiah1* and Ch. Venkata Ramana Reddy2
1Department of Chemistry, SAP College, Vikarabad -501 101 Ranga Reddy Dist. Telangana, India
2Department of Chemistry, JNTUH College of Engineering, Kukatpally, Hyderabad-500 085, Telangana, India.
Corresponding Author Email: tadooru_veeraiah@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/310360
Article Received on :
Article Accepted on :
Article Published : 20 Aug 2015
Siimple and sensitive extractive spectrophotometric methods have been described for the determination of Cyproheptadine hydrochloride (CPH) in pure form and in pharmaceutical formulations. The developed methods involved formation of coloured chloroform extractable ion-pair complexes of the CPH with triphenyl methane dyes viz., bromothymol blue (BTB), bromocresol green (BCG) and bromocresol purple (BCP) in acidic medium. The extracted complexes showed absorbance maxima at 419, 419 and 405nm with use of the above dyes, respectively. Beer’s law is obeyed in the concentration ranges 3.0-25, 2.5-22.5 and 1.5-25μg/ml with BTB, BCG and BCP respectively. The stoichiometry of the complex is found to be 1:1 in each case. The effect of concentration of dye, pH and interference of excipients have been studied and optimized. The limit of detection and limit of quantification have been determined for three methods. The methods are applied to the determination of CPH in commercial tablets and results of analysis are validated statistically through recovery studies also the methods are validated as per the guidelines of ICH.
KEYWORDS:Spectrophotometry; Cyproheptadine hydrochloride; Bromothymol blue; Bromocresol green; Bromocresol purple; Ion-pair complex; Validation
Download this article as:| Copy the following to cite this article: Sayanna, Veeraiah T, Reddy Ch. V. R. Determination of Cyproheptadine hydrochloride in Pure and Pharmaceutical forms : A Spectrophotometric study. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Sayanna, Veeraiah T, Reddy Ch. V. R. Determination of Cyproheptadine hydrochloride in Pure and Pharmaceutical forms : A Spectrophotometric study. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10347 |
Introduction
Cyproheptadine hydrochloride (CPH), chemically known as 4-(5H-dibenzo [a,d]cyclohepten-5-ylidene)-1-methylpiperidine hydrochloride (I), is an antihistaminic, anticholinergic and antiseretonergic agent. It also acts as a 5-HT2 receptor antagonist as well as blocking calcium channels1. Cyproheptadine is used to treat allergic reactions (specifically hay fever)2. Cyproheptadine has shown effectiveness in the treatment of nightmares including nightmares related to post traumatic stress disorder3,4. Cyproheptadine has been used in the management of moderate to severe cases of serotonin syndrome (a complex of symptoms associated with the use of serotonergic drugs, such as selective serotonin reuptake inhibitors and monoamine oxidase inhibitors)5-7.
 |
Scheme 1: Structure of Cyproheptadine hydrochloride Click here to View scheme |
Because of the physiological importance, Cyproheptadine (CPH) attracted the attention of analytical chemists for its qualitative and quantitative analysis. Quantitative determination of CPH is carried out using almost all physical methods. Chromatographic techniques such as gas chromatography8,9 and HPLC10-13 have been applied for the determination of CPH in biological fluids including serum, plasma and urine. Kashyap et al14 have described a derivative UV spectroscopic method for the determination of CPH in blood and tissue. A limited number of methods have been reported for the quantification of CPH in pharmaceutical dosage forms. GC15,16 and HPLC17-19 methods for the assay of CPH in dosage forms are found in the literature. Xhou20 reported a UV- spectrophotometric assay of CPH in compound tablets containing pemoline. Adanski21 was, perhaps, the first to report colorimetric method for the assay of CPH in tablets. The titrimetric methods22 were described for the determination of CPH in pharmaceuticals and the methods were based on the solvent extraction-titration of CPH with two ion association reagents, sodium lauryl sulphate, and tetraphenylborate. Shingbal and Naik23 have described a spectrophotometric method based on the complexation reaction. Based on ion-pair formation reactions, two cyproheptadinium-responsive electrodes were prepared with tetraphenylborate24 and p-dinonyl naphthalenesulphonic acid25 as ion-pair reagents and applied for the determination of CPH in pharmaceuticals with recoveries >98%. One titrimetric and two spectrophotometric methods26 are described for the determination of CPH in bulk drug and tablets employing N-bromosuccinimide as a brominating agent and two dyes, erioglaucine and meta-cresol purple as auxiliary reagents. Basavaiah et al27 developed two new procedures for the determination of CPH. The first method is based on the complexation of the drug with Bromophenol Blue (BPB) at pH 3.0 to form an ion-pair, which is extracted into chloroform and the absorbance measured at 420nm. The other method is based on the fact that CPH reacts with BPB under slightly altered conditions of pH, ionic strength and reagent concentration to form an insoluble ion-pair complex. The authors claimed that the methods developed are more simple and sensitive than many reported earlier. A sensitive method28 based on color development with citric acid-Ac2O reagent was described for the estimation of cyproheptadine in pharmaceutical dosage forms.
Although, UV-vis spectrophotometric methods for the determination of CPH are available, not much attention was paid to the development of spectrophotometric methods for its determination using dyes. Spectrophotometry is considered as the most convenient analytical technique because of its inherent simplicity, low cost and wide availability in most quality control laboratories. In continuation of our work on the spectrophotometric determination of drugs29, we report newly developed and validated spectrophotometric estimation methods of CPH in bulk and pharmaceutical formulations using triphenyl methane dyes viz., bromothymol blue (BTB), bromocresol green (BCG) and bromocresol purple (BCP). The developed methods involve formation of coloured chloroform extractable ion-pair complexes of the drug with CPH in acidic medium. The proposed methods are simple and sensitive besides being accurate and precise, and can be adopted by the pharmaceutical laboratories for quantitative analysis.
Experimental
Instruments
The UV-Vis. spectra of ion-pair complexes have been recorded on ELICO double beam SL 210 spectrophotometer using quartz cells of 10 mm path length. An Elico model Li-120 pH meter was used for pH measurement.
Materials
The drug, cyproheptadine hydrochloriede was procured from Hetero Drugs Private Limited, Hyderabad, as gift sample. HPLC grade chloroform and Analytical grade dyes viz., bromothymol blue, bromocresol green and bromocresol purple were used in the study. Other chemicals used in the study such as HCl, Sodium acetate are of AR grade and supplied by Sd Fine Chemicals, Mumbai.
Methods
The developed methods are based on the interaction of cyproheptadine hydrochloriede with triphenyl methane dyes viz., bromothymol blue (BTB), bromocresol green (BCG) and bromocresol purple (BCP) to form chloroform extractable ion pair complexes which showed absorbance maxima at 419, 419 and 405nm with use of above dyes respectively (Fig. 1a, 1b and 1c). The absorbance of this band increases with increasing the concentration of the drug and formed a basis for the quantification of the drug. The dyestuffs were used as 0.025% solutions in doubly distilled water. Sodium acetate-hydrochloric acid buffers of pH 2.8, 3.5 and 2.5 were prepared by mixing 50ml of 1.0M sodium acetate solution with calculated volume of 1.0 M HCl solution and diluted to 250 ml with doubly distilled water. The pH of each solution was adjusted to an appropriate value with the aid of a pH meter.
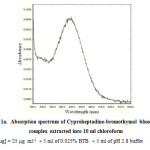 |
Figure 1A: Absorption spectrum of Cyproheptadine-bromothymol blue(BTB) complex extracted into 10 ml chloroform |
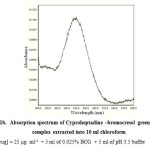 |
Figure 1B: Absorption spectrum of Cyproheptadine –bromocresol green (BCG) complex extracted into 10 ml chloroform Click here to View figure |
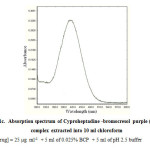 |
Figure 1c: Absorption spectrum of Cyproheptadine -bromocresol purple (BCP) complex extracted into 10 ml chloroform Click here to View figure |
Calibration Curves for the Methods
Different aliquots of CPH solution were transferred into 125 ml separating funnel. To this 5 ml of buffer, 5 ml of dye were added and total volume was made up to 20 ml with water. 10 ml of chloroform was added and the contents were shaken for 5 min. The two layers were allowed to separate for 5 min. The organic layer was separated and absorbance of yellow colored solution which is stable at least for 3 hrs is measured at 419 nm against blank similarly prepared. The same procedure of analysis is followed either for the determination of pure drug and for dosage form. The calibration graphs are linear for all the dyes analyzed (Fig. 2). The optical characteristics and statistical data for the regression equations for the proposed methods are presented in Table 1.
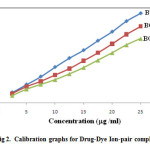 |
Figure 2: Calibration graphs for Drug-Dye Ion-pair complexes Click here to View figure |
Table 1: Optical characteristics and statistical analysis for the regression equation of the proposed methods
| Parameters | Extraction methods withb | ||
|
BTB |
BCG |
BCP |
|
| λmax (nm) |
419 |
419 |
405 |
| Beer’s law limit (μg ml-1) |
3.0-25 |
2.5-22.5 |
1.5-25 |
| Molar absorptivity (L mol-1 cm-1) |
16579 |
20067 |
1329 |
| Formation constant, K, M-1 |
1.25×106 |
1.58×106 |
1.65×106 |
| Sandell sensitivity (μg cm-2) |
0.0171 |
0.016 |
0.013 |
| Slope (specific absorptivity), b |
0.0584 |
0.06 |
0.0765 |
| Intercept (a) |
0.0267 |
-0.05 |
0.0077 |
| Correlation coefficient (r) |
0.997 |
0.993 |
0.9955 |
| Standard deviation of intercepts (% n=6) |
0.0021 |
0.0026 |
0.0012 |
| Limit of detection, μgml-1 |
0.406 |
0.246 |
1.12 |
| Limit of quantification, μgml-1 |
1.22 |
0.8 |
1.7 |
| Regression equationa |
Y=0.0584C± 0.0267 |
Y=0.06C± 0.05 |
Y=0.0765C± 0.0077 |
aWith respect to Y=bc+a, where C is the concentration (μg ml-1) and Y is absorbance
bSix replicate samples
Procedure for the Determination of Pure Drug
Five different solutions of pure drug in the range of calibration curve were selected and the recovery experiments were performed. The recoveries and their relative standard deviations, t-test and F-test are tabulated in Table 2.
Table 2: Application of proposed methods for the analysis of Cyproheptadine in pure form
|
Taken (μg ml-1) |
Proposed methods |
Reference |
|||||
|
Found (μg ml-1) |
Recovery (%) |
Method |
|||||
|
BTB |
BCG |
BCP |
BTB |
BCG |
BCP |
Recovery (%) |
|
|
3 |
3.05 |
2.96 |
3.02 |
101.66 |
98.66 |
100.66 |
99.70 |
|
6 |
5.93 |
6.09 |
6.01 |
98.83 |
101.5 |
100.16 |
101.30 |
|
9 |
9.10 |
9.09 |
8.95 |
101.11 |
101.00 |
99.44 |
101.60 |
|
12 |
11.97 |
12.09 |
12.06 |
99.75 |
100.75 |
100.50 |
101.10 |
|
15 |
14.98 |
15.04 |
14.98 |
99.86 |
100.26 |
99.86 |
101.23 |
|
99.98 |
|||||||
|
101.26 |
|||||||
|
99.96 |
|||||||
|
RSD (%) |
1.1319 |
1.0831 |
0.9398 |
0.7459 |
|||
|
Mean±SD |
100.24 ±1.1342 |
100.43 ±1.0878 |
99.94 ±0.936 |
100.76 ±0.7517 |
|||
|
t-test |
0.5932 |
0.5382 |
0.3294 |
||||
|
F-test |
0.4392 |
0.4774 |
0.6449 |
||||
Procedure for the Determination of Dosage forms
Eight tablets of Periactin 20mg each are powdered and dissolved in doubly distilled water and stirred thoroughly, filtered through a Whatman No. 42 filter paper. This solution was transferred into 100 ml standard volumetric flask and diluted with doubly distilled water as required. Different solutions of drug in the range of calibration curve were chosen and the assay was estimated using the calibration curve. The results of the recovery experiments are tabulated in Table 3.
Table 3: Application of proposed methods for the analysis of Cyproheptadine in pharmaceutical form
|
Taken (μg ml-1) |
Proposed methods |
Reference |
|||||
|
Found (μg ml-1) |
Recovery (%) |
Method |
|||||
|
BTB |
BCG |
BCP |
BTB |
BCG |
BCP |
Recovery (%) |
|
|
3 |
3.07 |
2.91 |
3.04 |
102.33 |
97.00 |
101.33 |
98.98 |
|
6 |
5.91 |
5.89 |
6.02 |
98.33 |
98.16 |
100.33 |
101.25 |
|
9 |
8.91 |
9.09 |
8.93 |
99.00 |
101.00 |
99.22 |
99.92 |
|
12 |
12.05 |
11.90 |
12.04 |
100.41 |
99.16 |
100.33 |
99.85 |
|
15 |
14.98 |
15.04 |
14.98 |
99.86 |
100.26 |
99.86 |
101.05 |
|
100.25 |
|||||||
|
99.98 |
|||||||
|
101.48 |
|||||||
|
RSD (%) |
1.5335 |
1.6150 |
0.7705 |
0.7186 |
|||
|
Mean±SD |
99.986 ±1.5333 |
99.116 ±1.6008 |
100.214 ±0.7722 |
100.345 ±0.7211 |
|||
|
t-test |
0.9889 |
1.0400 |
0.1052 |
||||
|
F-test |
0.2211 |
0.2029 |
0.8721 |
||||
Results and Discussion
Cyproheptadine forms ion-pair complexes in acidic buffer with triphenyl methane dyes viz., BTB, BCG and BCP. These complexes, extracted into chloroform absorbed maximally at 419 nm. The reagent blank under similar conditions showed no absorption. The methylated nitrogen of piperazine ring in Cyproheptadine is only the potential donor site and the protonation takes place at this site in acidic medium, while sulphonic acid group is present in any of the dyes that is the only group undergoing dissociation in the pH range 1-6. The colour of such dyes is due to the opening of lactoid ring and subsequent formation of quinoid group. It is supposed that the two tautomers are present in equilibrium but due to strong acidic nature of the sulphonic acid group, the quinoid body must predominate. Finally the protonated Cyproheptadine forms ion-pairs with the dyestuffs which is quantitatively extracted into chloroform (Scheme 1).
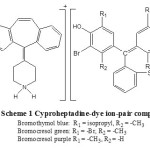 |
Scheme 1b Click here to View Scheme |
Stoichiometry
In order to establish stoichiometry between Cyproheptadine and dyestuffs used, the Job’s method of continuous variation has been applied30. In this method, solutions of CPH and dyestuff with identical molar concentrations [8 x 10-5M] were mixed in varying volume ratios in such a way that the total volume of each mixture was the same. The absorbance of each solution was measured and plotted against the mole fraction of the drug, [drug]/[drug]+[dyestuff]. This measurement showed that 1:1 complex was formed (Fig. 3).
![Fig 3. Continuous-variations study of drug-dye systems [Drug] = [Dye] = 8x10-5M](http://www.orientjchem.org/wp-content/uploads/2015/08/Vol31_No3_Dett_Syant_Fig3-150x150.jpg) |
Figure 3: Continuous-variations study of drug-dye systems [Drug] = [Dye] = 8×10-5M |
Optimization of the Factors Affecting The Absorbance
The influence of pH on the ion-pair formation of Cyproheptadine with dyestuffs used in the study has been carried out using sodium acetate-hydrochloric acid buffer. The results are shown in Fig.4. It is evident that absorbance of complexes with BTB, BCG and BCP was found to be constant within the pH ranges 2.2-3.5, 3.0-3.8 and 2.0-3.0 respectively. Thus, all the absorbance measurements were made at pH 2.8, 3.5 and 2.5 with BTB, BCG and BCP respectively.
![Fig. 4 Effect of pH [Drug] = [8µg ml-1], [Dye] = 5ml of 0.025%](http://www.orientjchem.org/wp-content/uploads/2015/08/Vol31_No3_Dett_Syant_Fig4-150x150.jpg) |
Figure 4: Effect of pH [Drug] = [8µg ml-1], [Dye] = 5ml of 0.025% |
The effect of concentrations dyestuff was also studied by adding different volumes of dyestuff to a constant amount of CPH (8 µg ml-1). It is apparent from Fig. 5 that the maximum absorbance, in each case, was found with 3.0 ml of dyestuff, beyond which absorbance was constant. Thus, 5 ml of each dyestuff was used for ion-pair formation throughout the experiment.
![Fig 5. Influence of the volume of 0.025% Dye [Drug] = [8µg ml-1]](http://www.orientjchem.org/wp-content/uploads/2015/08/Vol31_No3_Dett_Syant_Fig5-150x150.jpg) |
Figure 5: Influence of the volume of 0.025% Dye [Drug] = [8µg ml-1] |
A systematic study of the effect of foreign species present along with Cyproheptadine on its determination at 8 µg ml-1 levels was undertaken. This study was carried out by following the proposed procedures for a 10 ml sample system, by adding a known amount of foreign species to a Cyproheptadine solution of 8 µg ml-1. Table 4 summarizes the results obtained. However, the drug content from the powdered tablets was extracted into chloroform, which completely removes any interference by the common excipients found in formulations.
Table 4: Interference study
|
Sl. No |
Excipients
|
Tolerance limit (μg ml-1) |
|
1 |
Microcrystalline cellulose |
85 |
|
2 |
Starch |
134 |
|
3 |
Lactose |
125 |
|
4 |
Povidone |
58 |
|
5 |
Silicon dioxide |
80 |
|
6 |
Titanium dioxide |
55 |
Validation of the Proposed Method
All the proposed methods have been validated in terms of guideline proposed by International Conference on Harmonization31 viz. selectivity, specificity, accuracy, precision, limits of calibration curve, LOD, LOQ, robustness, ruggedness and regression equation. The student t-test and variance F-test have been performed in comparison with a reference method. Table 1 summarizes the values for Beer’s law limits, molar absorptivity, regression equation, correlation coefficients, relative standard deviation and recoveries. To test the reproducibility of the proposed methods, six replicate determinations of 8µg ml-1 of CPH were made. The coefficient of variation was found to be less than 1.5% for all the procedures.
The proposed methods have been successfully applied to the determination of Cyproheptadine in pharmaceutical preparations. The performance order of the proposed methods is BTB > BCG > BCP. The results obtained and shown in Table 2 and Table 3 were compared to those obtained by reference method30 by means of t-test at 95% confidence level. In all cases, the average results obtained by proposed methods and reference method were statistically identical, as the difference between the average values had no significance at 95% confidence level.
Conclusion
In conclusion, Cyproheptadine forms ion-pair complexes with acidic triphenylmethane dyes viz., bromothymol blue, bromocresol green and bromocresol purple and in 1:1 proportion. These complexes are extractable into chloroform and offer a basis for assay of the drug. The developed methods are simple, sensitive, reproducible and can be used for routine analysis of Cyproheptadine in pure and formulation forms.
Acknowledgements
The authors are thankful to Prof. G. Venkateshwarlu, Department of Chemistry, Osmania University, Hyderabad for helpful discussion and to Sri M. Ravindra Reddy, Chairman, Managing Committee, Dr.D.Dathatreya Reddy Principal and Dr. M. Chennaiah, HOD, Dept. of Chemistry of SAP College, Vikarabad for providing facilities. The authors are thankful to the UGC for financial assistance under Major Research Project.
References
- Lowe, D. A.; Matthews, E. K.; Richardson, B. P., British Journal of Pharmacology, 1981, 74 : 651-663.
- MedlinePlus Drug Information: Cyproheptadine.
- Rijnders, R. J. P.; Laman, D. M.; Van Diujn. H., American Journal of Psychiatry, 2000, 157 :1524-1525.
- Gupta, S.; Popli, A.; Bathurst, E.; Hennig, L.; Droney, T.; Keller, P., Comprehensive psychiatry, 1998, 39 : 160-164.
- Mills, K. C., American Family Physician, 1995, 52:1475-1482.
- Gillman, P. K., Journal of Psychopharmacology, 1999, 13 : 100-109.
- Hall, M.; Buckley, N., Australian Prescriber, 2003, 26 : 62-63.
- Hucker, H. B.; Hutt, J. E., J Pharm Sci, 1983, 72:1069-1070.
- Levinc, B.; Green Johnson. D.; Hogan. S.; Jesmialek, J. E., J Anal Toxicol, 1998, 33:73-74.
- Ng, L. L., J Chromatogr, 1983, 257 : 345-353.
- Novak, E. A.; Stanley. M.; Mc Intyre, J. M.; Hryhorczuk, L. M., J Chromatogr, 1985, 339 : 457-461.
- Foda, N. H.; Jun, H. W.; Mc Call, J. W., J Liq Chromatogr, 1986, 9 : 817-830.
- Kountourellis, J. E.; Ebete, K. O., J Chromatogr Biomed Appl, 1985, 664 : 468-471.
- Kashyap, R.; Iyer, L. R.; Singh, M. M., Indian J Forensic Sci, 1990, 4 : 203-216.
- Yang, C.; Men, Q., Yaowu Fenxi Zazhi, 1991, 11: 113-118.
- Sane, R. T.; Karkhanis, P. P.; Anaokar, P. G., Indian J Pharm Sci, 1981, 43 : 111-112.
- Rao, G. R.; Raghuveer, S., Indian Drugs,1985, 22 : 377-380.
- Burrows, G. W.; Alliger, C. L., J Pharm Sci, 1983, 72 : 1212-1213.
- Williams, P. A., Pharmacopoeial Forum, 1988, 14 : 3463-3472.
- Zhou. X., Zhongguo Yaoke Daxue Xuebao, 1989, 20 : 307-308.
- Adanski, S., Acta Pol Pharm, 1965, 22 : 311-314.
- Emmanuel, J. ; Yegnanarayan, T. V., Indian Drugs, 1982, 19 : 505-507.
- Shingbal, D. M.; Naik, S. D., Indian Drugs, 1981, 18 : 444-446.
- Issa, Y. M.; Rizk, M. S.; Mohammed, S. S., Anal Lett, 1992, 25 :1617-1629.
- Bunaciu, A. A.; Ioneseu, M. S.; Enacheseu. I.; Baiuleseu, G. E.; Cosofret, V. V., Analusis,1988, 16 : 131-134.
- Raghu, M. S.; Basavaiah, K., Chemical Industry & Chemical Engineering, 2012, 18(3) : 449-458.
- Basavaiah, K., Scienceasia, 2004, 30:163-170.
- Shingbal, D. M.; Naik, S.D., Indian J Pharm. Sci, 1982, 44(1) : 5-6.
- Ravi, M.; Veeraiah, T.; Venkata Ramana Reddy, Ch., Orient J Chem 2014, 30(2):723-730.
- Vosburgh, W.C.; Coopper, G.R.,. J Am Chem Soc, 1941, 63:437-442.
- ICH (International Conference on Harmonization) of Technical Requirement for the Registration of Pharmaceuticals for Human use, Validation of analytical procedures: definitions and Terminology Genera. 1996.

This work is licensed under a Creative Commons Attribution 4.0 International License.









