Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by Amino Acid Complexes
K. Kiruthikajothi*1, and G. Chandramohan2
1Research and Development Centre, Bharathiar University, Coimbatore – 641 046. India. 2Department of Chemistry, A.V.V.M. Sri Pushpam College, (Autonomous), Poondi, 613 503, Thanjavur, India. Corresponding Author Email: k.kiruthikajothi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310312
Article Received on :
Article Accepted on :
Article Published : 22 Aug 2015
Using the amino acids methionine and serine reduced Schiff base and their copper(II) complexes were synthesized. The inhibition effect of these copper (II) complexes on the corrosion of mild steel in 1 M HCl solution was investigated. The corrosion inhibition action is studied through weight loss method. Among the tested complexes [CuCl(SMet)PPh3.H2O] exhibited better corrosion inhibition at 3 mmol concentration. The adsorption of the complexes on the metal surface obeys Langmuir’s adsorption isotherm model.
KEYWORDS:Aminoacids; corrosion inhibition; Langmuir adsorption
Download this article as:| Copy the following to cite this article: Kiruthikajothi K, Chandramohan G. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by Amino Acid Complexes. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Kiruthikajothi K, Chandramohan G. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by Amino Acid Complexes. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10427 |
Introduction
When the metals are exposed to environment their surface begins to destructed or deteriorated by chemical or electrochemical reaction with its environment to attain a stable lower energy state is called corrosion [1]. It is a spontaneous, silent and slow process. The corrosion of metals is a worldwide scientific problem as it affects the metallurgical, chemical and oil industries [2, 3].
Corrosion may cause economic losses directly or indirectly. In India the cost of corrosion per annum is estimated around Rs.80000 crore i.e 6.1% GDP of the nation. The corrosion inhibition is necessary to reduce the unusual economical losses and to prevent the waste of resources during the industrial application [4,5].Corrosion inhibition is essential for extension of the durability of equipment and limiting dissolution of toxic metals from components in to environment.
Corrosion is classified in to two types such as chemical corrosion and electrochemical corrosion [6]. The corrosion inhibition method should be selected based upon the type and cause of corrosion. Among the various methods, the use of chemical inhibitors is the most efficient and economical method to reduce the corrosion of metals by forming phase layers on the metal surface or by adsorption [7]. Based on the mechanism inhibitors are classified in to three types such as form a protective film on the metal surface as type I and those which reduce the aggressiveness of the corrosive environment as type II and those which will form a protective film on the metal surface as well as reducing the aggressiveness of the corrosive environment as type III [8].
This is the recent concern for most researchers to develop cheap, non toxic corrosion inhibitors and minimize impact of the chemicals to nature, humans, aquatic life and other creatures [9]. Many of the current research have been geared to synthesize inhibitors using amino acids which are environmental friendly.
The objective of the present work is to study the inhibition of mild steel corrosion in 1 M HCl solution by copper (II) amino acid complexes using weight loss method.
Experimental
Synthesis of Cu(II) Complexes
Amino acid (10 mmol) (methionine or serine) was dissolved in 10 mL distilled water with KOH (0.56 g, 10 mmol). Salicylaldehyde (1.22 g, 10 mmol) dissolved in 10 mL ethanol was added to amino acid solution and stirred for 3 h at RT. It was cooled in an ice path, reduced with 5 mL of sodiumborohydride (0.378 g, 10 mmol) containing few drops of NaOH solution. The pH of the solution was adjusted to 3.5 – 6 using few drops of con HCl to obtain the solid precipitate. The obtained aminoacid ligand wad washed and dried in RT. Copper chloride dihydrate (1.70 g, 10 mmol) was dissolved in 15 mL ethanol. Pyridine or triphenylphosphine (0.79g or 2.62 g, 10 mmol) was dissolved in 10 mL ethanol and transferred to copper chloride solution. It was stirred for 10 minutes. The corresponding aminoacid ligand (10 mmol) was dissolved in 10 mL distilled water with KOH (1 mL, 0.01M) was added to it and allowed to stir for 2h at room temperature. The reaction mass was filtered and allowed to evaporate at RT [10].
The synthesized copper(II) complexes used as inhibitors are given below
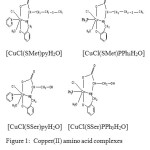 |
Figure 1: Copper(II) amino acid complexes used as inhibitors in this study |
Weight loss Measurements
A commercially available grade of mild steel coupons with size 4 cm × 1 cm × 0.1cm were used for weight loss measurements. These coupons were greased for preventing them from corrosion. Before use the studies, these coupons were degreased by acetone washed with double distilled water and then dried. The Analytical Reagent grade of HCl was used as corrosive medium. It was prepared by dilution of con HCl to 1 M HCl using distilled water. The specimens were containing a small hole in upper edge for hanging them into solution. The complexes were made in to 1mmol, 2mmol and 3mmol concentrations.
Results and Discussion
The corrosive medium 1 M HCl solution (100 mL) was taken in thirteen 250 mL beakers. One beaker was reserved as control that is without adding the inhibitor. Copper complex inhibitors at different concentrations 1 mmol, 2 mmol and 3 mmol were added to the remaining beakers. The mild steel specimens were immersed in to the solutions which are previously weighed. After three days the specimens were removed from the solutions, polished with emery papers, washed with distilled water, degreased with acetone, dried and reweighed. The weight loss, % efficiency and rate of corrosion are presented in table1.
Percentage of inhibition efficiency and corrosion rate are calculated using the following formulae.
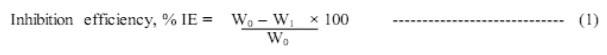
W0 – weight loss in the absence of inhibitor and W1 – weight loss in presence of inhibitor
The corrosion rate (in mpy-1 millimetre penetration per year) was expressed by the following equation
![]()
W – weight loss(g)
D – density of the specimen(7.85 g/cm3)
A – surface area of specimen(cm3)
t – immersion time(days)
Table 1: Percentage of inhibition efficiency and corrosion rate of copper(II) aminoacid complexes
| S.No | Name of the inhibitor | concentration | Control (W0) | Weight loss (W1)
|
% efficiency | Corrosion rate(mp/y) | |
| 1 | [CuCl(SMet)py.H2O] |
1 mmol 2 mmol 3 mmol |
0.2 | 0.110.08
0.07 |
45 60 65 |
0.62 0.45 0.39 |
|
| 2 | [CuCl(SMet)PPh3.H2O] |
1 mmol 2 mmol 3 mmol |
0.090.07
0.05 |
56 65 75 |
0.51 0.40 0.28 |
||
| 3 | [CuCl(SSer)py.H2O] |
1 mmol 2 mmol 3 mmol |
0.120.10
0.09 |
41 48 55 |
0.68 0.56 0.51 |
||
| 4 | [CuCl(SSer)PPh3.H2O] |
1 mmol 2 mmol 3 mmol |
0.110.09
0.08 |
45 54 60 |
0.62 0.51 0.45 |
||
From the results it was revealed that the corrosion inhibition efficiency increases and the corrosion rate decreases as the increase in inhibitor concentration. This is because of sufficient adsorption and wider coverage by the inhibitor molecules. All the complexes showed moderate inhibition in 3 mmol concentration and the order follows [CuCl(SMet)PPh3.H2O] > [CuCl(SMet)py.H2O] > [CuCl(SSer)PPh3.H2O] > [CuCl(SSer)py.H2O]. The π electrons of aromatic ring and the lone pair of electrons of hetero atoms in inhibitor molecules facilitate electrons transfer from the inhibitor to the metal and get adsorbed on the metal surface. The complex [CuCl(SMet)PPh3.H2O] showed the better inhibition when compared to other complexes. It showed maximum inhibition efficiency of 75% at 3 mmol concentration. This is because addition to N&O it contains S&P atoms and high molecular weight complex. So a widely spread film is formed on the metal surface and protect it from aggressive medium by blocking of active sites and thus decrease the corrosion rate.
Adsorption Isotherm
The surface coverage (θ) values of four complexes at three different concentrations were tested to various isotherms like Langmuir, Freundlich, Frumkin and Temkin adsorption isotherms. The plot of C/θ versus C gives the straight line and the data were best fitted with Langmuir adsorption isotherm.

C – Concentration of the corrosion inhibitor
θ – Degree of surface coverage
K – Adsorpion equilibrium constant
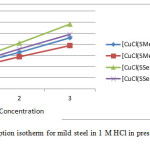 |
Figure 2: Langmuir’s adsorption isotherm for mild steel in 1 M HCl in presence of inhibitors at different concentrations Click here to View figure |
Conclusions
Four copper(II) amino acid complexes were synthesized and their corrosion inhibition action were investigated on mild steel corrosion in 1 M HCl by weight loss method. Methionine amino acid containing copper complexes showed better activity than amino acid serine containing copper complexes. The adsorption mechanism obeyed the Langmuir adsorption isotherm.
References
- Barouni, K., Kassale, A., Alburine, A., Jbara, O., Hammouti, B., Bazzi, L.,J.Mater. Environ. Sci.2014,5(2),456-463
- Simonovic, T., Petrovic, B., Radovanovic, B., Milic, M., Antonijevic, M. Chemical papers.2014, 68(3),362-371
- Hari Kumar, S., Karthikeyan, S. J. Mater. Environ.Sci.2012,3(5),925-934
- Zaafarany, I,A. Int.J.Electrochem.Sci.2013,8,9531-9542
- Afia, L., Salghi, R., Bammou, L., Bazzi,El., Hammouti, B., Bazzi, L., Bouyanzer, A. Journal of Saudi Chemical Society.2014, 18, 19-25
- Kanimozhi, S.A., Rajendran, S.Int. J. Electrochem. Sci., 2009,4, 353
- Prathibha, B.S., Kotteeswaran,P., bheema Raju, V. Res. J.Recent Sci.2013,2(4),1-10
- Cano,E., Bastidas,D.M., Simancas, J., Lopez Caballern, J.A., Bastidas,J.M. Adsorption Sci., and Tech.2004,22,565
- Akpan, A., Offiong, O., International journal of corrosion.2013,1-5
- Kiruthikajothi, K., Chandramohan, G., Chandraleka, S. Int J Curr Res. 2013, 5(11), 3419-3421.

This work is licensed under a Creative Commons Attribution 4.0 International License.









