Corrosion Inhibition Efficiency of Bio Waste on Mild Steel in Acid Media
T. Sathiyapriya1*, G. Rathika2
1SVS College of Engineering,Coimbatore – 642109,Tamilnadu,India. 2Department of Chemistry,PSG College of Arts and Science,Coimbatore – 641014, Correspondence Author Email: sathiyachallenge87@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310350
Article Received on :
Article Accepted on :
Article Published : 05 Sep 2015
The inhibitive action of alkali extract human hair extract (Bio waste) against the corrosion of mild steel (98.58%) in solutions of nitric acid has been studied using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). At inhibitor concentration range (0.004 % v/v to 0.1% v/v) in 0.3 M HNO3acid, the results showed that these compounds suppressed both cathodic and anodic processes of mild steel corrosion in 0.3 M HNO3by adsorption on the mild steel surface. The efficiency of the inhibitor in both potentiodynamic and EIS measurements reveal that these compounds inhibit the mild steel corrosion in 0.3 M HNO3and that the efficiency increases with increasing of the inhibitor concentration. Scanning electron microscopy (SEM) study confirmed that the inhibition of corrosion of mild steel is through adsorption of the extract molecules on surface of metal.
KEYWORDS:Mild steel; EIS; SEM; Polarization; Acid inhibition
Download this article as:| Copy the following to cite this article: Sathiyapriya T, Rathika G. Corrosion Inhibition Efficiency of Bio Waste on Mild Steel in Acid Media. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Sathiyapriya T, Rathika G. Corrosion Inhibition Efficiency of Bio Waste on Mild Steel in Acid Media. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10562 |
Introduction
Corrosion is the disintegration of an engineering material into its constituent atom due to chemical reaction with its surroundings or electrochemical oxidation of metal in reaction with an oxidant such as oxygen.(1)The field of corrosion becomes more important because it creates economic losses when referring to cost of design, manufacturing, construction and management.(2)The problem of corrosion inhibition lies on the use of corrosion inhibitors, which help to increase the life span of the materials.
Mild steel is most widely used alloy in industries due to its excellent mechanical properties and low cost, since the beginning of industrial revolution. The acid media is used in the study of corrosion of mild steel has become important because of its industrial applications such as acid pickling, industrial cleaning, acid descaling, and oil well acid in oil recovery and petrochemical processes.(3)To avoid the corrosion of base metal, chemical inhibitors are often used to control the metal dissolution. The most commonly used acid corrosion inhibitors are the heterocylic compounds containing Nitrogen, Sulphur and Oxygen atom and multiple bonds.(4, 5, 6)
Most of the organic and inorganic compounds are expensive and toxic to both humanbeings.(7)This led investigations to focus on the development of non-toxin, eco-friendly inhibitors. Previously numerous works were done on the use of plant extract as corrosion inhibitors. The aim of this present work is to study the inhibitory action of human hair on the surface of mild steel using Weight loss, Potentiodynamic polarization technique and Electrochemical impedance spectroscopy.
Human hair is a material which considered useless in most societies and so it is found in the municipal waste streams in almost all cities and towns of the world. (8)
In rural areas with low population density, the hair is thrown away in nature where it slowly decomposes over several years eventually returning the constituent elements, namely, carbon, nitrogen, sulfur, and so forth, to their respective cycles. In urban areas with high population density, it often accumulates in large amounts in the solid waste streams and chokes the drainage systems, causing a multifaceted problem. Due to slow degradation, it stays in the dumps and occupies more volume of space over time; leachate from these dumps increases the nitrogen concentration in the water bodies, causing problems of Eutrophication. Burning of human hair produces foul odour and toxic gases such as ammonia, carbonyl sulphides, hydrogen sulphides, sulphur dioxide, phenols, nitriles, pyrroles and pyridines.(9)
The best way to solve such problems is to develop systems which utilize the waste material as a resource. In addition to reducing waste, it contributes to the economy.
Constituents of Hair
Human hair consists of proteins, lipids, water, trace elements and pigments. (10)Experimental results show that the different ethnic origins of head hair samples constitute different amounts of amino acids. They are listed below: (11-12)
- Alanine
- Arginine
- Aspartic acid
- Cystine
- Glutamic acid
- Glycine
- Histidine
- Isoleucine
- Leucine
- Lysine
- Methaionine
- Phenylalanine
- Prolyne
- Serine
- Threonine
- Tryptophan
- Tyrosine
- Valine
Materials and Methods
Preparation of Human Hair Extract
Ethnic hair samples were collected and cut into small pieces (1cm length), washed with double distilled water and then ethanol and dried well. 10 ml of 1M NaOH is taken in a clean 1000 ml beaker and to that 10 g of hair sample is digested at 70-90o C. After digestion it is made upto 500 ml using distilled water and thus 2 % stock solution of the inhibitor was prepared.
Preparation of Specimens
Mild steel plates of the dimensions 2.5 cm × 1 cm × 0.1 cm have been used. It was polished using 1/0, 2/0, 3/0, and 4/0 emery papers, washed with double distilled water, dried, and finally degreased with the acetone.
Electrolyte
The electrolyte of 0.3M HNO3 solution was prepared by diluting Conc. HNO3 using double distilled water which is used as a corrosive solution.
Weight loss Measurements
Gravimetric measurements were performed on mild steel coupons with the above mentioned dimensions in 0.3 MHNO3 solution with and without of inhibitors. Every sample was weighted by an electronic balance, and thenplaced in the acid solution (100 mL). The duration of the immersion was 1 hr, 3 hr, 5 hr, 7 hr and 24 hr at room temperature. After immersion, the surface of the specimens was cleaned by distilled water, dried and weighed in order to calculate the corrosion rate (CR) and inhibition efficiencies (%). For each experiment, a freshly prepared solution was used. The corrosion rate of mild steel (CR) and the inhibition efficiency (IE %) of the inhibitor and were calculated using the following equations:
![]()
Where W is weight loss in mg, D is density in mg, A is area of exposure in cm2, and T is time in hours. Inhibition efficiency has been determined by using the following relationship:
![]()
W0 is weight loss without inhibitor. Wt is weight loss with inhibitor.The effects of temperature on the corrosion inhibition performance for the various concentrations of the human hair extract were studied in the range of (303 to 323 K). The solution temperature was thermostatically controlled at desired temperatures.
Electrochemical Measurements
Electrochemical studies were carried out by using an electrochemical work station, ivium instrument. A three electrode compartment cell was used for the electrochemical measurements. A saturated calomel electrode (SCE) and a platinum electrode were used as the reference and the counter electrode, respectively. The potentiodynamic current potential curves were recorded by polarizing the specimen to −250 mV cathodically and +250mV anodically with respect to OCP at a scan rate of 1 mV∙s−1. Electrode potentials were measured with respect to SCE. The polarization studies were done immediately after the EIS studies on the same electrode without any further surface treatment. The AC impedance was performed in the frequency range from 20 kHz to 200 Hz with single amplitude of 10 mV.
Surface Analysis
The surface morphology of mild steel specimen immersed in 1M HNO3 in the absence and presence of hair sample extract at room temperature for 2 hr was studied using a Scanning Electron Microscope (SEM).
Results and Discussion
Effect of Inhibitor Concentration
The corrosion of mild steel in 0.3 M HNO3 in the absence and presence of various concentrations of hair sample extract was determined for different immersion periods (1 hr, 3 hr, 5 hr, 7 hr and 24 hr) at room temperature. From the weight loss data, plot of Inhibition efficiency Vs Concentration and Corrosion rate Vs time were made and shown in Fig. 1 and 2. It was observed from the plots that the IE increases with increase in hair sample extract. Maximum inhibition efficiency of 98.95% was obtained at 0.1% v/v hair sample extract concentration at room temperature for 1 hr immersion.
It is observed from the plot of CR Vs immersion time (Fig. 2) shows that at room temperature, CR of mild steel decreased on increasing hair sample extract concentration. This behavior could be attributed to the increase in adsorption of the amino acids present in the extract at the metal solution interface on increasing its concentration.
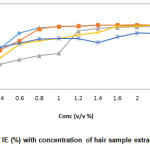 |
Figure 1: Variation of IE (%) with concentration of hair sample extract in 0.3 M HNO3 Click here to View figure |
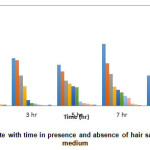 |
Figure 2: Effect of Corrosion rate with time in presence and absence of hair sample extract in 0.3 M HNO3 medium Click here to View figure |
Effect of Temperature
The influence of temperature on the corrosion of mild steel in 0.3 M HNO3 without and with the presence of human hair extract was studied using weight loss method in the temperature range of 303 K -323K with 1 hr immersion. Table 1 shows the values of corrosion rate and inhibition efficiency obtained from weight loss measurements at various temperatures mentioned below. The variation of CR of mild steel as a function of different concentrations of human hair extract is shown in Fig. 3. The data in table 1 shows the CR and I.E (%) values with and without inhibitor at different temperatures for 1 hrit is seen that the Corrosion rate increases with increase in temperature in both uninhibited and inhibited solutions.
Table 1: CR and IE (%) of sample extract ((2%) in 0.2M NaOH) in 0.3N HNO3 at different temperatures (immersion time-1 hr)
|
Conc (ml) |
Wt. loss |
303K |
Wt. loss |
313K |
Wt. loss |
323K |
Wt. loss |
333K |
||||
|
CR |
IE% |
CR |
IE% |
CR |
IE% |
CR |
IE% |
|||||
|
Blank |
0.141 |
0.5325 |
** |
0.16 |
0.6043 |
** |
0.189 |
0.7138 |
** |
0.201 |
0.7591 |
** |
|
0.6 |
0.019 |
0.0718 |
86.52 |
0.12 |
0.4419 |
26.88 |
0.138 |
0.5212 |
26.98 |
0.102 |
0.3852 |
49.25 |
|
0.8 |
0.008 |
0.0302 |
94.33 |
0.08 |
0.2984 |
50.63 |
0.141 |
0.5325 |
25.40 |
0.13 |
0.4910 |
35.32 |
|
1.0 |
0.006 |
0.0227 |
95.74 |
0.04 |
0.1360 |
77.50 |
0.122 |
0.4608 |
35.45 |
0.104 |
0.3928 |
48.26 |
|
1.2 |
0.004 |
0.0151 |
97.16 |
0.02 |
0.0680 |
88.75 |
0.084 |
0.3172 |
55.56 |
0.11 |
0.4154 |
45.27 |
|
1.4 |
0.002 |
0.0076 |
98.58 |
0.01 |
0.0415 |
93.13 |
0.054 |
0.2039 |
71.43 |
0.055 |
0.2077 |
72.64 |
|
1.6 |
0.002 |
0.0076 |
98.58 |
0.01 |
0.0227 |
96.25 |
0.018 |
0.0680 |
90.48 |
0.087 |
0.3286 |
56.72 |
This can be attributed to the fact that the rate of corrosion reaction increases with increase in temperature. But the rate of corrosion is less in inhibited solutions compared with uninhibited solutions. Decrease in inhibition efficiency with increase in temperature is suggestive of physical adsorption mechanism (13) and may be attributed to increase in the solubility of the protective films and of any reaction products precipitated on the surface of mild steel that may otherwise inhibit the corrosion process. As the temperature increases the number of adsorbed molecules decreases, leading to a decrease in the inhibition efficiency. A decrease in inhibition efficiency with the increase in temperature in this case may also be due to weakening of physical adsorption. (14)
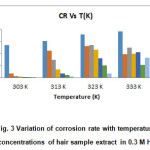 |
Figure 3: Variation of corrosion rate with temperature at different concentrations of hair sample extract in 0.3 M HNO3 medium Click here to View figure |
Thermodynamic Parameters
The activation energy Ea was calculated from the slope obtained by plotting log Corrosion rate Vs 1/T by Arrhenius equation. From the table 2Ea in the presence of the inhibitors are higher than those in the uninhibited acid solution. These results indicate the adsorption is by physical adsorption mechanism. (15)
Activation parameters ∆H, ∆S for the mild steel in 0.3M HNO3were calculated from the plot of log CR/T Vs 1/T. ∆H is calculated from the slope using the formula
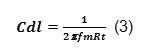
∆S is calculated from the intercept using the formula
![]()
Table 2 enumerated the values ∆Hads and ∆Sads at different concentrations of inhibitors. ∆Hads and ∆Sads were found negative in all the concentrations of the inhibitor showing that the reactions are exothermic.
Table 2: Activation parameters of HNO3 on mild steel surface in both uninhibited and inhibited solutions.
|
Conc % (v/v) |
Ea |
∆Hads |
∆Sads |
|
Blank |
11.89995 |
-42.095 |
-178.306 |
|
0.012 |
81.35621 |
-273.996 |
-177.588 |
|
0.016 |
117.4486 |
-392.938 |
-177.219 |
|
0.02 |
122.7715 |
-411.759 |
-177.169 |
|
0.024 |
123.8246 |
-418.365 |
-177.161 |
|
0.028 |
134.1066 |
-455.989 |
-177.056 |
|
0.032 |
89.35971 |
-317.44 |
-177.514 |
Potentiodynamic Polarisation Measurements
The potentiodynamic anodic and cathodic polarization plots for mild steel specimen in 0.3 M HNO3 solution with and without the sample extract at different concentrations were given in Fig.4. The respective electrochemical parameters current density (Icorr) Corrosion potential (Ecorr) Cathodic Tafel slope(βc) anodic Tafel slope (βa) I.E(%) is given in table 3.
Table 3: Polarization parameters for mild steel in 0.3 M HNO3 in the absence and presence of different concentrations of the inhibitor
|
S.No. |
Conc. |
Ecorr V |
Icorr A/cm^2 |
βa V/dec |
βc V/dec |
C. Rate mm/y |
Rp Ohm |
IE% |
|
|
Icorr |
Rp |
||||||||
|
1 |
BLANK |
-0.4237 |
0.001643 |
0.111 |
0.128 |
5.378 |
15.77 |
** |
** |
|
2 |
0.012 |
-0.4938 |
0.000417 |
0.121 |
0.092 |
1.364 |
54.39 |
74.63 |
71.01 |
|
3 |
0.016 |
-0.4803 |
0.00032 |
0.1 |
0.12 |
1.046 |
74.3 |
80.55 |
78.78 |
|
4 |
0.02 |
-0.4946 |
0.000247 |
0.085 |
0.144 |
0.808 |
93.81 |
84.97 |
83.19 |
|
5 |
0.024 |
-0.4849 |
0.000236 |
0.096 |
0.133 |
0.7711 |
102.9 |
855.65 |
84.67 |
|
6 |
0.028 |
-0.4908 |
0.000142 |
0.079 |
0.138 |
0.4658 |
153.4 |
91.33 |
89.72 |
It is evident from the polarisation result, the Icorr decreases and I.E(%) increases with increasing concentration. This confirms the corrosion inhibition action of hair sample. Moreover the cathodic and anodic Tafel slope values changed with the inhibitor concentration indicating that the hair sample extract controlled both the cathodic hydrogen evolution and anodic mild steel dissolution reactions.
Ecorr value shifted towards more negative potential. It has been reported that a compound can be classified as an anodic and cathodic type. As the inhibitor concentration increased, the corrosion rate value decreased, which again a supporting evidence for the inhibiting action of the inhibitor.
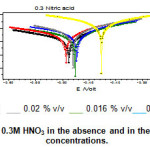 |
Figure 4: Tafel plots of mild steel in 0.3M HNO3 in the absence and in the presence of inhibitor at different concentrations. |
Electrochemical Impedance Spectroscopy
Fig.8 represents the electrochemical impedance EIS Nyquist plots for mild steel in 0.3M HNO3 with and without concentrations of hair sample extract at 298 K. The electrochemical Impedance parameters derived from these investigations are mentioned in table 4.
Table 4: Impedence parameters for mild steel in 0.3 M HNO3in theabsence and presence of various concentration of inhibitor
|
S.No. |
Conc. |
Cdl |
Rct |
IE% |
|
|
Cdl |
Rct |
||||
|
1 |
BLANK |
5.19*10^-5 |
4 |
** |
** |
|
2 |
0.012 |
4.40*10^-5 |
25.2 |
15.22 |
84.13 |
|
3 |
0.016 |
4.38*10^-5 |
25.5 |
15.61 |
84.31 |
|
4 |
0.02 |
2.62*10^-5 |
26.4 |
49.52 |
84.85 |
|
5 |
0.024 |
2.71*10^-5 |
33.6 |
47.78 |
88.10 |
|
6 |
0.028 |
2.64*10^-5 |
43 |
49.13 |
90.70 |
The double layer capacitance (Cdl) values were obtained at maximum frequency (fmax), at which the imaginary component of the Nyquist plot is maximum and calculated using the following equation:
As it is evident from Fig.5 impedance diagrams, show a semi-circle appearance, indicating a charge transfer process mainly controls the corrosion process. From the impedance data, we notice an increase in charge transfer and decrease in double layer capacitance with increasing inhibitor concentration indicate that the hair sample extract inhibits the corrosion rate of mild steel by an adsorption mechanism. The decrease in the Cdl value suggests that the inhibitor molecules function by adsorption at the metal/solution interface as they displace the water molecules and other ions originally adsorbed on the surface leading to the formation of a protective adsorption layer on the electrode surface which increases the thickness of the electrical double layer. The thickness of this protective layer is related to Cdl in accordance with Helmholtz model given by the equation:
Where Σ is the dielectric constant of the medium and Σo is the permittivity of free space (8.854*10-14 F/cm) and A is the effective surface area of the electrode. From the equation, it is clear that as the thickness of the protective layer that is, the film formed by inhibitor molecules, increases the Cdl should decrease. Here the Cdl value was found to be highest for uninhibited solution and as the inhibitor concentration increases the Cdl value decreases.
 |
Figure5: Nyquist plots for mild steel in 0.3 MHNO3 in the absence and presence of various concentrations of inhibitor. Click here to View figure |
The charge transfer resistance Rct values are calculated from the difference in impedance at low and high frequencies. The Rct value is a measure of electron transfer across the mild steel surface and it is inversely proportional to the corrosion rate. The inhibitor efficiency of inhibitors for the corrosion of mild steel in 0.3 M medium is calculated using Rct values as follows:
![]()
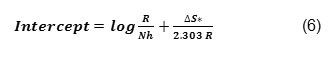
Where Rct(o) and Rct(i) are the charge transfer resistance values in absence and presence of inhibitor respectively. The Rct value was found to be highest for the unhibited solution.
![]()
The inhibitor efficiencies obtained from Rct are in good agreement with those obtained from potentiodynamic polarization and weight loss measurements.
Corrosion Surface Morphology
Fig. 9 (a) and (b) presents image of the corrosion morphology of mild steel immersed in 0.3M HNO3 and 0.3M HNO3 with 0.032 % v/v extract after 2 hours of immersion. The nitric acid aggressively corroded the sample in Fig. (a) causing a rougher surface , whereas the sample shown in Fig. 9(b) with smoother surface is noticed. The mild steel with smooth surface was attributed to the fact that the hair sample extract to form an adsorbed film on the surface of the mild steel, which is absent in Fig.9(a) mild steel.
 |
Figure 6: (a) and 6(b) Corrosion surface morphology |
This result supplements the results of electrochemical techniques and confirms that the extract of human hair inhibited corrosion or mild steel through adsorption of the inhibitor molecules on metal surface.
Conclusion
- The I.E (%) of hair sample extract on corrosion of mild steel in 0.3M HNO3 solution increases in increase in concentration of the inhibitor. The effect of temperature indicates that the I.E (%) deceases with rise in temperature.
- The increase in activation energies of corrosion process in presence of the extract indicates that the inhibitor indicates that the amino acids in the hair extract retards the rate of corrosion of mild steel in 0.3M HNO3
- The phenomenon of physical adsorption is proposed from the activation and thermodynamic parameters obtained.
- EIS measurement reveals that charge transfer resistance increases with increase in concentration. This result is correlating with the result of polarization measurement.
- EM study confirm that the inhibition of corrosion of mild steel is through adsorption of the extract on surface of metal and these studies also supplement the results of electrochemical techniques.
References
- Saedah R. Al-Mhyawi; Orient. J. Chem.,2014,30(2),541-552
- Iloamaeke I. M.;Onuegbu T. U.; Umeobika U. C.;Umedum N. L.;International Journal of Science and Modern Engineering,2013,1,48-52
- Singh A.K.; QuraishiM.A.;Corro. Sci., 2010,54,152-160
- DonerA.;SolmazR.;OzcanM.; KardasG.; Corro. Sci., 2011,50,2902-2913
- TaoZ.; ZhangS.; LiW.;HouB.; Ind. Eng. Chem.,2011,50,6082-6088
- LebriniM.; RobertF.;VezinH.;RoosC.;Corro. Sci.,2010,52,3367-3376
- KiruthikajothiK.;ChandramohanG.;Jayabharathi G.;Chem. Sci. Rev.Lett., 2014,3,603-607
- KumarS.; BhattacharyaI. K.; VaidyaA. N.;ChakrabartiT.;DevottaS.;Akolkar A. B.;WasteManage.,2009,29,883-895
- BrebuM.;SpiridonI.;J. Anal. Appl. Pyrol.,2011,91,288-295
- Block R. J.; Boiling D.; The amino acid composition of proteins and foods,2nded.; Charles C.Thomas, Springfield, Ill., (1951)
- Gold R. J; Scriver C.R.;Clin. Chim. Acta, 1971,33,465
- Ari L. Horvath;TheScientificWorlsJOURNAL,2009,9,255-271
- Dasami P.M.; Parameshwari K.; Chitra S.;Orient. J. Chem.,2015,31,85-191
- ObotI.B.; Obi-EgbediN.O.;UmorenS.A.; Int. J. Electrochem. Sci., 2009,4, 863-877
- Saedarrosion Surface Morphology:

This work is licensed under a Creative Commons Attribution 4.0 International License.









