Bases of Technology and Optimal Regime Indicators of Acid Decomposition Process of Phosphorus Sludge
U. B. Nazarbek1*, U. Besterekov1, I. A. Petropavlovsky2, S. P. Nazarbekova3, O. K. Beisenbayev1
1Department of Chemical technology of inorganic chemistry, M.Auezov South Kazakhstan State University, Shymkent, Kazakhstan
2Department of Chemical technology of inorganic chemistry, D.Mendelyev Russia Chemical and technological University, Moscow, Russia
3Department of Chemistry, M.Auezov South Kazakhstan State University, Shymkent, Kazakhstan.
Corresponding Author Email :-unazarbek@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/310319
Article Received on :
Article Accepted on :
Article Published : 04 Aug 2015
The bases of technology and optimal regime parameters of the process of acid decomposition of phosphorus sludge are presented in this paper. The chemistry of acid decomposition of phosphorus sludge is practically similar to the decomposition of natural phosphorite. Two-stage acid decomposition phosphorus sludge can be considered as the most technologically method of processing it into phosphorus fertilizer. Results of studies allowed to establish as a optimal regime parameters of acid decomposition of phosphorus sludge make the following parameters: temperature of the decomposition process - 600°C; process duration - 60 minutes; acid concentration - 50%; consumption of acid - 70% of the stoichiometric requirement.
KEYWORDS:phosphorus sludge; sulphuric acid decomposition; superphosphate; Gibbs’s energy
Download this article as:| Copy the following to cite this article: Nazarbek U. B, Besterekov U, Petropavlovsky I. A, Nazarbekova S. P, Beisenbayev O. K. Bases of Technology and Optimal Regime Indicators of Acid Decomposition Process of Phosphorus Sludge. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Nazarbek U. B, Besterekov U, Petropavlovsky I. A, Nazarbekova S. P, Beisenbayev O. K. Bases of Technology and Optimal Regime Indicators of Acid Decomposition Process of Phosphorus Sludge. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10157 |
Introduction
The full scientifically grounded processing technologies of phosphorus sludge into demanded products, containing phosphorus are still lacking for today. The known technological solutions based on the acid decomposition of phosphorus sludge, in spite of their rather important applied significance, are still not out of the stage of laboratory tests. The main reasons of such situation are the lack of scientific substantiation of their technological bases, lack of reliable experimental results about the regime technological parameters of the acid decomposition process of phosphorus sludge.
The full scientific substantiation of technology of acid processing of phosphorus sludge on the target products of fertilizing destination and the establishment of the optimal parameters of the process by temperature, duration of the acid treatment, concentration and acid consumption are an actual problem.
There are some scientific works, devoted to research compounds of phosphorus for example, in article1 the anodic solution of copper phosphide is used for obtaining trialkylphosphate in alcohol solutions, which copper phosphide is a product, obtained at the processing of wastes of phosphorus production. Ways of obtaining tri-n-butylphosphate and tri-izo-butylphosphate are presented. In article2 was described the synthesis and characterization of phosphine and arsine complexes of ruthenium (II & III) ligated with 3-(4-pyridyl)-4-substituted-triazoline-5-thione. Organometallic complexes of ruthenium (II & III) with the formula [RuH(CO)(EΦ3)2L] and [RuCl2(EΦ3)2L] (E = P/As; L = deprotonated mononegative bidentate 3-(4-pyridyl)-triazoline-5-thione and its 4-phenyl substituted derivative) were synthesized and characterized by elemental analysis, physico-chemical and spectroscopic methods. All new compounds were iso-structural with precursor complexes.
Material and Methods
Previous studies have shown3 that phosphorus sludge of large-tonnage waste of phosphorus production is a valuable secondary phosphate raw material. It was established that the average content of total P2O5 is varied in the range of 10-17%. Including the assimilable P2O5 is 5-7%, and water soluble – 2.4%3. The given indicators suggest that this kind of industrial wastes is quite suitable for processing into phosphorus fertilizer – simple superphosphate, if to use the fundamental principles of double superphosphate production technology4-8.
In this, the process flow scheme of acid decomposition of phosphorus sludge into the desired product can be represented in a simplified form in the following manner (Figure 1):
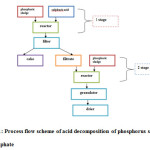 |
Figure1: Process flow scheme of acid decomposition of phosphorus sludge into superphosphate Click here to View figure |
As previously mentioned3, phosphorus sludge is similar with raw material – phosphate rock by chemical composition. Therefore, the chemistry of the main process of stages of sulphuric acid decomposition of phosphorus sludge can be described by the known mechanism as follows:
Ca10(PO4)6F2 + 10H2SO4 + 20H2O = 6H3PO4 + 10CaSO4 × 2H2O + 2HF (1)
During the first stage of the process, impurities that present in phosphorus sludge also enter into the chemical reaction with sulphuric acid. As stated previously3, a considerable amount of silicon, magnesium, calcium and potassium compounds in carbonate and oxide forms are contained in the composition of phosphorus sludge. Therefore, the chemistry of secondary processes that take place during sulphuric acid decomposition of phosphoric sludge, can be described as follows:
СаСО3 + H2SO4 + H2O = CaSO4 × 2H2O + СО2 (2)
MgСО3+ H2SO4 + H2O =MgSO4 + СО2+ 2H2O (3)
K2O + H2SO4 = К2SO4 + H2O (4)
Silicon oxide, contained in phosphorus sludge, interacts with hydrofluoric acid, obtained as a result of decomposition of the basic reaction (1), forming a gaseous SiF4, as follows:
SiO2 + 4HF = SiF4 + 2H2O (5)
Preliminary laboratory studies showed that the main products of the sulphuric acid decomposition stage of phosphorus sludge are phosphoric acid and poorly soluble sulphates of calcium, magnesium, potassium, and therefore the product of sulphuric acid decomposition stage is presented as suspension.
The results of the subsequent filtration separation of the suspension showed that the liquid phase filtrate of phosphoric acid has a concentration of about 17-19% and the solid residue on the filter is a cake, consisting of a mixture of sulphate compounds.
The filtered phosphoric acid at the second stage of acid decomposition process was used for the decomposition of a new portion of phosphorus sludge in order to obtain the final product of superphosphate.
The chemistry of this process can be described as follows:
Ca10(PO4)6F2 + 14H3РO4 + 10H2O = 10Ca(H2PO4)2 × H2O + 2HF (6)
During the second stage of the process, impurities that present in phosphoric sludge, also enter into a chemical interaction with phosphoric acid:
СаСО3 + 2H3РO4 = Ca(Н2РO4)2 × H2O + СО2 (7)
MgСО3+ 2H3РO4 = Mg (Н2РO4)2 × H2O + СО2 (8)
3K2O + 2H3РO4 = 2К3PO4 + 3H2O (9)
SiO2 + 4HF = SiF4 + 2H2O (10)
The main product of the final stage of phosphoric acid decomposition of phosphorus sludge is presented as a monocalcium phosphate monohydrate – superphosphate. Here, water-soluble phosphates of potassium and magnesium in a digestible form were formed in small amounts. The final product was obtained as a plastic mass of dark gray color that readily amenable to granulation and drying.
Results and Discussion
In the given paper presents the results of research, carried out in order to establish the optimal regime indicators of stage of sulphuric acid decomposition of phosphoric sludge.
The targeted studies began with a study of the temperature regime of the process of acid decomposition of phosphoric sludge. It is known that the rate of acid decomposition process of phosphate rock depends upon the nature and is directly proportional to the surface of the mineral grains. The smaller the grain size is, the faster and more efficiently the decomposition process is9. As stated previously, phosphorus sludge by nature is close to phosphorite, and the main part of its accounts for the fine fraction with a particle size of 0,100-0,315 mm10 by granulometric composition. In order to determinate the temperature regime of phosphorus sludge decomposition process as an initial experimental data of known base data, the least indicators by duration of the acid decomposition and concentration of sulphuric acid, respectively, 60 minutes and 50% were taken.
About the results of temperature effect on degree of decomposition of the studies object were judged by P2O5 – common, assimilable and water soluble in the final product of decomposition, obtained as a result of sulphuric acid decomposition of the first portion and following phosphate treatment of a new portion of phosphoric sludge. In this, for obtaining phosphoric acid, which necessary for use on the second stage of acid decomposition, a suspension product of the first stage of sulphuric acid decomposition of phosphorus sludge was subjected to filtration with the separation of the residual solid phase in the form of cake, which the composition was also analyzed by total P2O5.
The results of studies are presented in Figure 2.
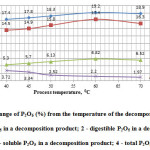 |
Figure2: Change of P2O5 (%) from the temperature of the decomposition process: 1 – total P2O5 in a decomposition product; 2 – digestible P2O5 in a decomposition product; 3 – soluble P2O5 in a decomposition product; 4 – total P2O5 in a cake. |
From the data in Figure 2 shows that in the range of temperature from 40 to 700°C with increasing temperature, degree of decomposition phosphorus sludge increases, reaching a peak at the temperature of 600°C, as evidently enough demonstrate the data by P2O5 in the final product and in the cake.
Figure 3 shows the temperature dependence of the Gibbs energy change in the main reactions of acid decomposition and degree of decomposition of phosphoric sludge.
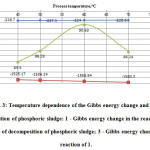 |
Figure3: Temperature dependence of the Gibbs energy change and degree of decomposition of phosphoric sludge: 1 – Gibbs energy change in the reaction of 6; 2 – degree of decomposition of phosphoric sludge; 3 – Gibbs energy change in the reaction of 1. |
As seen in Figure 3, the whole temperature range of 300 – 700 °C the studied process spontaneously takes place in conceived direction. This is evidenced by the negative values of Gibbs free energy change, calculated by the method of Temkin – Shvartsman11-15. However, as follows from the data in Figure 3, the maximum degree of decomposition of phosphorus sludge is achieved only at temperatures of the order 600C or not higher. At temperatures above 600C, acid decomposition process decreases, it is explained by the beginning of gelling of a mixture in the reactor16-17.
Thus, the data of figures 2 and 3 clearly indicate that the optimal temperature regime of acid decomposition of phosphorus sludge is 60°C.
Fig. 4 shows the results of studies of the influence of the process duration on the efficiency of phosphorus sludge acid decomposition at the optimum temperature of 600C. In this, the concentration of sulphuric acid, as in the previous experiments, was taken equal to 50%.
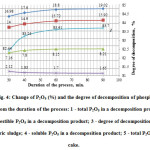 |
Figure4: Change of P2O5 (%) and the degree of decomposition of phosphorus sludge from the duration of the process: 1 – total P2O5 in a decomposition product; 2 – digestible P2O5 in a decomposition product; 3 – degree of decomposition of phosphoric sludge; 4 – soluble P2O5 in a decomposition product; 5 – total P2O5 in the cake. Click here to View figure |
As follows from the data in Fig. 4, in the range of from 30 to 90 minutes the degree of decomposition phosphorus sludge increases, reaching 83.61% of a maximum for 60 minutes. Further increase of the process duration does not lead to a substantial increase of the decomposition degree.
Figure 5 shows the research results of the character of changes of phosphorus sludge acid decomposition process rate depending on the process duration.
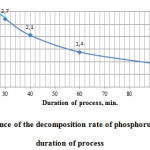 |
Figure5: Dependence of the decomposition rate of phosphorus sludge from the duration of process |
As follows from the data in Fig. 5, the acid decomposition process of phosphorus sludge consists of two stages. The first stage during the interval from 20 to 25 minutes phosphorus sludge is advantageously subjected to physical dissolution in sulphuric acid. Acid decomposition process of phosphorus sludge with transfer of considerable part of P2O5 total phosphorus sludge into assimilable form most effectively begins with 25 minutes.
The rate of the second phase of the process in time is reduced. After 60 minutes at the rate of the process of 1.4 g/min (Fig. 5) the maximum degree of decomposition is 83.61% of phosphorus sludge (Fig. 4).
Thus, based on the data Figures 4 and 5, we can conclude that the optimal duration of the acid decomposition process of phosphorus sludge makes 60 minutes.
During experimentally found optimal operating parameters of acid decomposition of phosphorus sludge on temperature (600C) and duration (60 minutes) were performed overestimation of influence of the acid concentration on the process. The results are shown in Fig. 6.
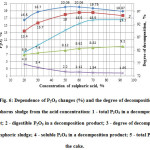 |
Figure6: Dependence of P2O5 changes (%) and the degree of decomposition of phosphorus sludge from the acid concentration: 1 – total P2O5 in a decomposition product; 2 – digestible P2O5 in a decomposition product; 3 – degree of decomposition of phosphoric sludge; 4 – soluble P2O5 in a decomposition product; 5 – total P2O5 in the cake. Click here to View figure |
Fig. 6 shows that the acid concentration has a significant impact on the process of decomposition of phosphoric sludge. With increasing acid concentration the degree of decomposition of phosphorus sludge increases, reaching a maximum degree of transfer of total P2O5 of phosphorus sludge into digestible form of P2O5 in the final product at sulfuric acid concentration of 50%.
Thus, optimal concentration of sulphuric acid for decomposition of phosphorus sludge should be reasonably taken as 50%.
It was established that the degree of sulfuric acid decomposition of phosphorus sludge depends on the flow rate of acid. Fig. 7 shows the results of the research dependence of P2O5 changes (%) of the acid flow rate. At this the acid flow rate is represented in percentage of its stoichiometric requirement, calculated by CaO the formula of n = 98×c/56. Where, c- the installed content of CaO in phosphorus sludge3, 98 and 56 – molecular weights of sulphuric acid and calcium oxide, respectively.
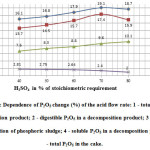 |
Figure7: Dependence of P2O5 change (%) of the acid flow rate: 1 – total P2O5 in a decomposition product; 2 – digestible P2O5 in a decomposition product; 3 – degree of decomposition of phosphoric sludge; 4 – soluble P2O5 in a decomposition product; 5 – total P2O5 in the cake. Click here to View figure |
Fig. 7 shows, the maximum degree of transfer of total P2O5 of phosphorus sludge onto digestible form of P2O5 in the final product is achieved at a flow rate of sulfuric acid of 70% of the stoichiometric requirement. This is understandable, since the crystal structure of phosphoric sludge, which is a waste of phosphorus production by electrothermal method, is less stable than natural phosphate rock and phosphoric sludge, is predominantly consisting of particles with a size of 0,100-0,315 mm, and significantly differs from the latter by the degree of dispersion.
Conclusion
Thus, we conclude:
- the two-stage acid decomposition of phosphorus sludge may be considered as one of the most technologically advanced ways of processing it into fertilizer, containing phosphorus;
- chemistry of decomposition process of phosphorus sludge does not significantly differ from the processes, taking place during the acid decomposition of natural phosphorite;
- taking into account the structural and composite and dispersion features of the object of research, the following indicators are quite reasonable take as an optimal regime parameters of stages of sulfuric acid decomposition of phosphoric sludge: temperature of the decomposition process – 600; duration of the process – 60 minutes; concentration of the acid – 50%; 50% of acid flow rate – 70% of the stoichiometric requirement.
- unlike natural phosphorite, sulphuric acid decomposition of phosphorus sludge flows at the lowest energy and material costs by acid.
References
- Aueshov, A.P.; Satayev S.; Tukibayeva A.S., Orien. J.Chem., 2014, 30(4), 1553-1556
- Pandey, R.N.; Nisha Kumari, P. Orien. J.Chem., 2014, 30(4), 2105-2111
- Nazarbek, U.B.; Besterek, U.B.; Petropavlovskiy I.A. and Pochitalkina, I.A. Chemical Industry of Today, 2014, 8, 33-38
- Aldashov B.A. and Lisitsa V.I., Disposal of Karatau phosphorite wastes – the path to a competitive economy and improve the environment, Gylym. Almaty, (2007)
- Dzhusipbekov U.ZH., Chernjakova R.M., Oshakbayev M.T. and Nurgaliyeva G.O., Processing of substandard phosphorites of Karatau and technogenic wastes on fertilizers, Gylym. Almaty, (2000)
- Batkaev R.I., Development of technology for obtaining marketable products from technogenic wastes of production of phosphorus: Author’s abstract of Doctoral thesis, M. Auezov South Kazakhstan State University. Shymkent, (2010)
- Beisenbayev O.K. Report of the scientific – research work “Development of technology for obtaining complex fertilizers, meliorants, structure formers of soils based on modified polyampholytes with controlled properties, synthesized using a secondary acrylate containing and hydrocarbon raw materials”, Shymkent, (2013)
- Nazarbek, U.B.; Besterek, U.; Nazarbekova, S.; Beisenbayev O.K.; Kydyralieva, A.D. Chem. J. Kazakhstan, 2014, 4, 111-115
- Kopylov B.A., Technology of extraction phosphoric acid, Chemistry. Leningrad, (1972)
- Nazarbek, U.B.; Besterek, U.; Petropavlovsky I.A.; Nazarbekova, S.P. Chem. J. Kazakhstan, 2014, 2, 164-168
- Besterek U., Theoretical bases of technology of inorganic substances, SKSU named after M. Auezov. Shymkent, (2014)
- Alekseev A.I., Kulinich O.V., Ramzanova L.P., Yuzvyak S., Thermodynamic analysis of reactions in chemical technology, SZTU. Sankt- Peterburg, (2003)
- Karapentyants M.H., Chemical Thermodynamics, Chemistry. Moscow, (1975)
- Ryabin V.A., Ostroumov M.A., Sweet T.F., Thermodynamic properties of substances, Chemistry. Leningrad, (1977)
- Ravdel A.A., Ponamareva A.M., Quick Reference on physico-chemical values, Chemistry. Leningrad, (1983)
- Yakhontova E.L., Petropavlovsky I.A., et al, Acid methods of processing of phosphate raw material, Chemistry. Moscow, (1988)
- Petropavlovsky, I.A.; Pochitalkina, I.A.; Kiselev, V.G.; Ahnazarova S.L.; Myrzahmetova, B.B. Chem. Eng., 2012, 8, 453

This work is licensed under a Creative Commons Attribution 4.0 International License.









