Anticancer and antiviral estimation of three Ulmus pravifolia extracts and their chemical constituents
Manal M.Hamed1, Samir M. El-Amin1, Laila A. Refahy1*, El-Sayed A. Soliman2, Wafaa A. Mansour3, Hoda M. Abu Taleb4 and Eman A. Morsi1
1Medicinal chemistry department, Theodor Bilharz Research Institute, Giza, Egypt
2Chemistry Departement, Faculty of Science, Ain Shams University, Cairo, Egypt
3Immunology Depatment, Theodor Bilharz Research Institute, Giza, Egypt
4Departement of Environmental Research, Theodor Bilharz Research Institute, Giza, Egypt.
Corresponding Author: lailarefahy@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310341
Article Received on :
Article Accepted on :
Article Published : 22 Jul 2015
Anticancer and antiviral activity of methanol, chloroform and butanol extracts of Ulmus pravifolia were tested on three cytokines of the human ascites fluid, TNF-alpha, Interferons-gamma and NO. Chloroform and butanol extracts showed a significant curative effect. Both butanol and chloroform extract undergo chromatographic fractionation to yield five compounds identified as Hederagenin 3-O-ß-D-glucopyranoside (1), Kaempferol 3-O-ß-D-glucopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-O-ß-D-glucopyranosyl-7-O-ß-D-glucopyranoside (2), 24-hydroxy-24-methylcycloartanol trans-ferulate (3), lupeol caffeate (4) and Ulmicin D (5). All compounds were isolated from the plant for the first time. The five compounds showed significant curative effect.
KEYWORDS:Ulmus pravfolia; antiviral; anticancer; cytokines; hepatitis C; hepatocellular carcinoma; TNF-alpha; Interferon-gamma; NO
Download this article as:| Copy the following to cite this article: Hamed M. M, El-Amin S. M, Refahy L. A, Soliman E. A, Mansour W. A, Taleb H. M. A, Morsi E. A. Anticancer and antiviral estimation of three Ulmus pravifolia extracts and their chemical constituents |
| Copy the following to cite this URL: Hamed M. M, El-Amin S. M, Refahy L. A, Soliman E. A, Mansour W. A, Taleb H. M. A, Morsi E. A. Anticancer and antiviral estimation of three Ulmus pravifolia extracts and their chemical constituents. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=9875 |
Introduction
Cancer is the abnormal growth of cells that can lead to death. According to the American Cancer Society, deaths arising from cancer constitute 2–3% of the annual deaths recorded worldwide. Although there are many kinds of cancer, they all have in common this out-of-control growth of cells (1). Hepatocellular carcinoma (HCC) is a primary hepatic cancer, being a common cancer type worldwide. Chronic infection with hepatitis B (HBV) and C viruses (HCV) often results in cirrhosis and enhances the probability of developing hepatocellular carcinoma (2). Because of high death rate associated with cancer and because of the serious side effects of chemotherapy and radiation therapy, many cancer patients seek alternative and/or complementary methods of treatment. Recently, some plants caused a significant effect on tumor cells (3), also more than 50% of all modern drugs in clinical use are of natural products, many of which have the ability to control cancer cells (4).
Cytokines is a large group of extracellular protein or glycoproteins used in the treatment of cancer, immune disorders and various other related diseases. Much of their therapeutic effect relies on direct influence of immune activity. They can have an effect not only on cells in the close proximity but also those in distant organs. Cytokines are characterized by a considerable complexity of actions such as redundancy, pleiotropy, multi functionality, synergistic or antagonistic effects and cascades of positive or negative feedbacks. At present, more than 100 different cytokines are known (5).
Elms are deciduous and semi-deciduoustrees comprising the genus Ulmus (family Ulmaceae). Ulmaceae is a family of trees and shrubs comprising 19 genera and about 2000 species occurring in both temperate and tropical climates (6). Ulmus genes having excellent effect on rheumatoid arthritis, metastasis of cancer (7,8) and inflammation (9). Polysaccharides isolated from plants belonging to this genus used as an effective component for the treatment of cancer, glycosuria, AIDS, pathogenic virus diseases, anti-inflammatory and an immune-reinforcing agent (10,11). Polyphenols, leucoanthocyanins and (+)-catechin (12), flavonoids, proanthocyanidins (13,14), mucilage, tannins, lignans, aromatic sequiterpenes, sterols, and triterpenes (15) were isolated from elm species. In previous work the methanol extract of Ulmus parvifolia showed cytotoxic effect against both myeloma and HepG2 cell line (16).
The aim of this work is to study anticancer and antiviral activities of three extracts of Ulmus parvifolia and the isolated compounds on three cytokines of the human ascites fluid, TNF-alpha, Interferons-gamma and NO. The structure of all compounds isolated from U. pravifolia, were determined by different spectroscopic techniqes including 1H, 13CNMR and mass spectra as well as by chemical analysis.
Experimental
Plant Material
Leaves and stems of Ulmus pravifolia (Ulmaceae) were collected in October from El-Orman garden, Gize, Egypt. Identification of plant was confirmed by Mrs. Treaze Labib, High specialist of Plant Taxonomy, Department of Flora and Taxonomy, El-Orman Garden, Giza, Egypt. Vouchers specimens of this plant were kept in laboratory of Medicinal Chemistry, Theodor Bilharz Research Institute, Giza, Egypt.
Equipments
Melting points were determined by an electrothermal apparatus (Electrothermal 9200), Glass plates of different dimensions (TLC), Glass columns; 170 X 7 cm, 125 X 5 cm, Portable UV lamp Vilber Lourmat (VL-6 LC 254 and 365 nm) for the detection of fluorescent spots, Rotatory evaporator (Buchi, Rotavapor, R., Switzerland), ESI-MS was performed on a Micromass Q-TOF Micro instrument, 1HNMR (300, DMSO-d6) and 13C-NMR (75 MHz) were recorded on Varian Mercury 300 and JEOL-GX-500 spectrometer. The chemical shifts are expressed in δ (ppm) with reference TMS and coupling constant (J) in Hertz, Microplate reader (El 311 Microplate Autoreader, Bio-Tek Instruments, Burlington, VT) and Microplate washer.
Chemicals
Phosphate Buffered saline tab, Flavin Adenine Dinucleotide, Nicotinamide Adenine Dinucleotide Phosphate, Nitrate reductase (from Aspergillus species). Human TNF-alpha ELISA Kit and IFN-gamma ELISA Kit were purchased from Koma Biotech Inc. (Korea), sulphanilamide and N-(1-naphthyl) ethylendiamine. Sephadex LH-20 (Sigma), Silica gel 60 GF254 (Merck) for TLC, Silica gel (70-230 mesh) (Merck) for column chromatography and Whatmann filter paper No.1 mm and 3 mm for paper chromatography (Maidstone, England). Sulphuric acid (40%), Aniline phthalate reagent, Aluminum chloride and Ferric Chloride. Ascites fluid, Pure deionized water, 0.31M PBS (1 tab PBS+62 ml pure water). Sulphanilamide and N-(1-naphthyl) ethylendiamine in 5% H3PO4, phosphate Buffered saline (Sigma), FAD (Flavin Adenine Dinucleotide) (sigma), NADPH (Nicotinamide Adenine Dinucleotide Phosphate) (Sigma).
Extraction and isolation
2Kg of air-dried powder of the leaves of Ulmus parvifolia was extracted by 85% aqueous methanol (30 liter) under reflux (11 times, 6 weeks). The methanolic extract was evaporated till dryness using rotatory evaporator to give 366 gram which was dissolved in H2O, then partitioned with petroleum ether (60-80), chloroform, ethyl acetate and n-butanol. The yield of each extract was recorded as petroleum ether extract (12.2 g), chloroform extract (28.7 g), ethyl acetate extract (10.5 g) and butanol extract (98 g).
Chloroform extract (28.7 gm) was washed by charcoal to remove chlorophyll dyes to obtain chloroform extract (13 g), then submitted to column chromatography (125 X 5 cm) packed with silica gel (70-230 mesh, Fluka) as stationary phase which was eluted using solvent systems with increasing polarity starting from petroleum ether, petroleum ether: chloroform, chloroform, chloroform:methanol with different ratios increasing from 0-100. The collected fractions were concentrated and examined by TLC (Silica gel, solvent system CHCl3 and CHCl3:MeOH) and PC using two solvent systems (n-BuOH:AcOH:H2O, 4:1:5) and (AcOH:H2O, 85:15). Two compounds [1 & 5] were isolated from chloroform extract.
The dried precipitate of n-butanol extract (48 gm) was chromatographed on silica gel column which constructed by packing a glass column (170 x7cm) with silica gel adopting the wet method using methanol. The dried extract was adsorbed on 30 gm silica gel applied on the top of the prepared column. Elution started with petroleum ether (60-80 °C) followed by chloroform, and chloroform:methanol ratio increasing from 0-100. The elutes were collected in fractions each 250 ml which concentrated and examined by TLC and paper chromatography. Four compounds [1-4] were isolated from BuOH extract.
Acid hydrolysis of compound 1
20 mg of compound 1 was hydrolyzed using 4N HCl (10 ml) and methanol (5 ml) on boiling water bath for 4 hours. The reaction mixture was concentrated under reduced pressure to remove the methanol. The mixture was partitioned between chloroform and water using separating funnel, the chloroform extract was evaporated under reduced pressure and crystallized to give the aglycone which was identified by TLC analysis with authentic samples using solvent system [C6H6:MeOH, 80:20]. The aqueous layer was neutralized with NaHCO3, filtered and concentrated then the sugar was extracted with pyridine. The sugar obtained was compared with authentic sugar on TLC silica gel plate, with system [EtOAc:MeOH:AcOH:H2O, 13:4:3:3]. Moreover, identification of the sugar was further confirmed by PC (Whatmann) filter paper No. 1, by using solvent system [n- BuOH:AcOH:H2O, 4:1:5]. Spots were detected by spraying with a solution of aniline phthalate (freshly prepared).
Acid hydrolysis of compound 2
Three mg of compound 2 was hydrolyzed with 10% HCl (3.5 ml) in aqueous methanol at 80 oC for 2 hour, after the removal of the solvent the reaction mixture was diluted by distilled water, whereby it give aglycone and sugar, the aglycone was identified via CO-PC with authentic sample and sugar moieties were detected via CO-TLC with authentic sugar markers in system (CHCl3:Me2CO:MeOH:H2O, 3:3:3:1 and CH2Cl2:MeOH:H2O, 6:9:1) respectively.
Patients
Two patients 52 and 63 years old referred to the inpatient Tropical medicine department of Theodor Bilharz Research Institute (TBRI) were included in this study. The first patient was diagnosed with liver cirrhosis and hepatitis C (HCV), and the second patient was diagnosed with liver cirrhosis and hepatitis C (HCV), hepatocellular carcinoma (HCC). The two patients were subjected to clinical examination, detailed history taking, cystoscopy, biochemical investigations and biopsy.
Application of U. pravfolia extracts to ascetic fluid culture
- The culture of ascetic cells according to Kohler and Milstein (1975) (17). The ascetic fluids were collected from TBRI inpatient unit for patients whom suffered from HCV and HCV with cancer. The collected ascetic fluids were centrifuged, supernatants were decanted, finally pellets were taken and re-suspended in 1 ml complete culture growth medium (SCM).
- Preparation of ascetic fluids complete medium
- Reagents
Fetal calf serum (FCS) (Hyclone, Logan, Utah, USA).HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Seromed, Biochrome KG, Berlin, Germany).RPMI (Rose well Park Memorial Institute), 1640 medium (Sigma), Chem. Co, St Louis, MO, USA. Sodium bicarbonate, Penicillin- Streptomycin (P/S), 10000 unites (Sigma). L-glutamine (Gibco Lab, Grand Island, NY, USA).
Method
Serum free medium prepared from RPMI 1640 medium PH 7.2-7.4 was supplemented with 20 ml/ l HEPES, 3 ml/l sodium bicarbonate 7.5%, 10 ml/l P/S (10000 units) and 10 ml/l L-glutamine. Fetal calf serum (FCS) was heat inactivated at 56 for 30 min and 20% FCS were added to SFM. The ascetic cells were cultured in growth medium (consisting of FCS and SFM at about 1:10 ratio) with starting cultures of 1X106 cells/ml in 24 wells culture plates. 100 µg of each plant extract and 50 µg of each pure compound were dissolved in serum free medium. The different plant extracts were applied to the cultured ascetic cells and dropped as 500 ul in each well and Tissue culture plates were incubated for 24 hours at 37 oC in humidified atmosphere with 5% CO2. After this primary incubation period, 0.5 ml aliquot from each well was removed by aspiration and kept in vials at – 20 oC then after another 24 hours incubation at 37 oC humidified atmosphere with 5% CO2 and aliquot of 0.5 ml was aspirated in vials and freezed at -20 oC. Immunological parameters were applied for NO, IFN-γ, TNF-α concentration in ascetic fluids before and after 24 hours and 48 hours incubation with plant extracts different dilutions, also without plant application as control samples (18).
Nitrite assay
The ascites fluid samples were taken, collected within sterile tubes. Nitric oxide was assayed in patients’ ascites according to Tracey et al., (1995). NO concentrations were determined using the Griess reaction (19) with the following modification. Six µl of ascites fluid were mixed with 44 µl pure water, 20 µl 0.31M PBS, 10 µl 0.86 mM NADPH, 10 µl 0.11 mM FAD, 10 µl Nitrate reductase (1µ\ml) in individual wells for 1hour at room temperature in dark. 100 µl Griess reagent and 20 µ Nitrate reductase from Aspergillus species was added to determine the concentration of both nitrites and nitrates in ascites (µM/ml), since the enzyme reduces nitrates to nitrites. Absorbance was measured at 540 nm using a Microplate reader and converted to NO concentrations by using the following equation:
Concentration = Optical Density Χ 200 (ng/ml) (constant)
Gamma interferon assay
Interferon gamma was applied in patient ascites according to De maeyer et al., (1992) (20), by using IFN-gamma enzyme immunoassay kit process (KOMA BIOTECH INC. Korea). The plate washed three times using 300 ul of washing solution per well, the plate inverted to remove residual solution. 100 ul of sample added to well, covered and incubated at room temperature for 2 hours. 100 ul of the diluted detection antibody (0.25 ug/ml) added per well. 100 ul of the diluted Color Development Enzyme (1:20) added per well, incubated for 30 min. at room temperature. 100 ul of Color Development solution added to each well, incubated at room temperature for a proper color development (5-15 min).To stop the color reaction; 100 ul of the stop solution added to each well. the absorbance was read using a microtiter plate reader, at 450 nm wave length.
Tumor necrosis factor alpha assay
Tumor necrosis factor-alpha was applied in patient asciets according to Damas et al., (1989) (21) by using IFN-gamma enzyme immunoassay kit (KOMA BIOTECH INC. Korea). The plate washed using 300 ul of washing solution per well three times, then 100 ul of sample added to well, covered with sealer and incubated at room temperature for 2 hours. 100 ul of the diluted detection antibody (0.25 ug/ml) added per well, covered and incubated at room temperature for 2 hours. 100 ul of the diluted Color Development Enzyme (1:20) added per well. 100 ul of Color Development solution added to each well, incubated at room temperature for a proper color development (5-15 min). To stop the color reaction; 100 µl of the stop solution added to each well. The absorbance was read by using a microtiter plate reader at 450 nm wavelength.
Statistical analysis
Statistical analysis was performed using SPSS version 20 software. Results, presented as mean ± standard error, The Shapiro-Wilk test was used to assess whether data were normally distributed. To compare the mean values, a one-way analysis of variance (ANOVA) with post-hoc Bonferroni correction to account for multiple tests was used. The data were considered significant when p < 0.05.
Results and Discussion
Hederagenin 3-O-ß-D-glucopyranoside (1)
white solid, m.p 251-253 oC, Rf 0.67 [CHCl3 : MeOH, 9:1], +Ve ESI-MS: m/e 633.8 [M+H]+, 471 [(M+H)-162]+, 1HNMR: six methyls at δ 0.91 (3H, s), 0.76 (3H, s), 0.78 (s, 3H),1.11 (3H, s), 0.63 (3H, s), 0.71 (3H, s), 5.29 (olefinic proton) and 4.80 (1H, d, J=7.8 Hz anomeric proton). 13CNMR: (Table 1).
Kaempferol 3-O-ß-D-glucopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-ß-D-glucopyranos-yl-7-O-ß-D-glucopyranoside (2)
yellow powder, m.p 237-239 oC, Rf 0.32, (BuOH : AcOH:H2O, 4:1:1), 1H NMR: δ 4.5 (1H, brs, H-1” Rhamnose), 4.89 (1H, d, J=6.5 Hz, H-1′ Glc), 4.71 (1H, d, J=6.5 Hz, H-1”’ Glc), 4.2 (1H, d, J=6.5 Hz, H-1””-Glc) , 7.0 (1H, d, J=7.5 Hz, H-3′,5′), 7.8 (1H, d, J=7.5 Hz, H-2′, 6′), 7.09 (1H, d, J= 2.0 Hz, H-6) and 7.4 ( 1H, d, J=2.0 Hz, H-8). +Ve ESI-MS: m/e 881.73 [M+H]+, 718 [(M+H)-162]+, 572 [(M+H)-162+146)]+, 249 [(M+H) – (3 x 162+146)]+ .13CNMR: (Table 1).
24-hydroxy-24-methylcycloartanol trans-ferulate (3)
yellow powder, m.p 277-279 oC, Rf 0.51, (CHCl3:MeOH, 8:2) TLC, Rf 0.6 [BuOH : AcOH :H2O, 4:1:5] and Rf 0.23, [15% AcOH] on PC. 1HNMR: 6 methyls at δ (0.82-0.12), methylene protons δ (1.2- 1.6), OMe at δ 3.1 and aromatic protons at δ (7.66-7.69). -Ve ESI-MS: m/e 633 [M-H]–, 618 [M-H-Me]–, 531[M-H-(Me + C5H11O)]–, 502 (base beak) [M-H-(Me + C7H16O)]–.
Lupeol caffeate (4)
yellow powder, m.p 222-223 oC, Rf 0.5, [BuOH:AcOH:H2O, 4:1:5] and Rf 0.8, [15% AcOH], on PC. 1HNMR: six methyl groups at δ 0.83, 0.84, 0.85, 1.21, 1.20, 1.47, isopropyl group at δ 1.87, 4.17, 4.95 and caffeate moiety at δ 6.60, 6.67, 6.95, 7.25, 7.34. – Ve ESI-MS: m/e 587 [M-H]–, 423.27 [M-H-caffeate moiety]–.
Ulmicin D (5)
white powder, m.p134-136 oC, Rf 0.57, [CHCl3], 1H NMR: Six methyl groups at δ 0.60, 0.69, 0.80, 0.85, 0.89, 0.98, isopropylene group at δ 1.45, 4.30, 4.10, olifinic protons at δ 5.10, 7.8 (2H, dd, J=8.5, 1.8 Hz, H-6′, 6”), 7.7, 7.6 (2H, d, J=2.0 Hz, H-2′, 2”), 6.75 (2H, d, J=8.5 Hz, H-5′,5”), 5.4 (1H, m, H-11), 5.7 (1H, dd, J=10.0, 5.0 Hz, H-15) and 3.7, 3.8 (6 H, s (2OMe). +Ve ESI- MS: m/e 761.87 [M+2H]+.13C-NMR (Table1).
|
Compound 1 |
Compound 2 |
Compound 5 |
|||
|
Position |
δC(ppm) |
Position |
δC(ppm) |
Position |
δC (ppm) |
|
1 |
38.8 |
2 |
157.5 |
1 |
41.28 |
|
2 |
28.3 |
3 |
136.8 |
2 |
27.87 |
|
3 |
77.4 |
4 |
177.3 |
3 |
78.59 |
|
4 |
45.6 |
5 |
162.0 |
4 |
39.67 |
|
5 |
42.3 |
6 |
100.0 |
5 |
55.06 |
|
6 |
19.1 |
7 |
164.0 |
6 |
18.79 |
|
7 |
33.8 |
8 |
93.9 |
7 |
37.75 |
|
8 |
40.5 |
9 |
159.2 |
8 |
45.65 |
|
9 |
50.1 |
10 |
107.6 |
9 |
53.31 |
|
10 |
36.2 |
1′ |
122.1 |
10 |
39.23 |
|
11 |
23.1 |
2′ |
130.7 |
11 |
73.42 |
|
12 |
121.7 |
3′ |
115.9 |
12 |
31.35 |
|
13 |
140.9 |
4′ |
161.2 |
13 |
47.58 |
|
14 |
40.3 |
5′ |
116.0 |
14 |
46.36 |
|
15 |
29.2 |
6′ |
132.1 |
15 |
73.16 |
|
16 |
24.4 |
7-Glc |
16 |
39.71 |
|
|
17 |
45.6 |
1′ |
99.5 |
17 |
44.34 |
|
18 |
40.1 |
2′ |
85.5 |
18 |
51.56 |
|
19 |
45.6 |
3′ |
78.7 |
19 |
47.58 |
|
20 |
33.8 |
4′ |
71.1 |
20 |
147.59 |
|
Compound 1 |
Compound 2 |
Compound 5 |
|||
|
Position |
δC(ppm) |
Position |
δC(ppm) |
Position |
δC(ppm) |
|
21 |
38.8 |
5′ |
77.1 |
21 |
28.13 |
|
22 |
31.9 |
6′ |
62.2 |
22 |
32.67 |
|
23 |
61.5 |
3-Rha |
|
23 |
28.61 |
|
24 |
12.1 |
1″ |
103.5 |
24 |
15.74 |
|
25 |
19.1 |
2″ |
71.1 |
25 |
16.78 |
|
26 |
19.4 |
3″ |
70.1 |
26 |
19.10 |
|
27 |
25.9 |
4″ |
72.2 |
27 |
12.34 |
|
28 |
175 |
5″ |
68.8 |
28 |
14.88 |
|
29 |
33.8 |
6″ |
19.5 |
29 |
110.34 |
|
30 |
24.4 |
3-O-Glc |
30 |
20.05 |
|
|
3-O-Glc |
|
1‴ |
105.8 |
1′ |
123.47 |
|
1 |
101.3 |
2‴ |
78.4 |
2′ |
112.31 |
|
2 |
73.9 |
3‴ |
78.1 |
3′ |
146.61 |
|
3 |
77.4 |
4‴ |
70.1 |
4′ |
150.40 |
|
4 |
70.5 |
5‴ |
75.7 |
5′ |
113.98 |
|
5 |
77.1 |
6‴ |
61.6 |
6′ |
124.72 |
|
6 |
61.5 |
3-Glc |
7′ |
165.73 |
|
|
1‴′ |
102.6 |
1′′ |
123.40 |
||
|
2‴′ |
74.8 |
2′′ |
111.31 |
||
|
3‴′ |
77.1 |
3′′ |
146.52 |
||
|
Compound 1 |
Compound 2 |
Compound 5 |
|||
|
Position |
δC(ppm) |
Position |
δC(ppm) |
Position |
δC(ppm) |
|
4‴′ |
69.9 |
4′′ |
151.36 |
||
|
5‴′ |
75.5 |
5′′ |
114.54 |
||
|
6‴′ |
60.9 |
6′′ |
123.16 |
||
|
7′′ |
165.13 |
||||
Table2: Effect of different extracts of Ulmus parvifolia on the cytokines of the human ascites fluid TNF–α and IFN-γ after 24 and 48 hours
|
IFN-γ in HCV case (24 h) |
IFN-γ in malignant HCV case (24 h) |
TNF–α in HCVcase (24 h) |
TNF–α in malignant HCV case (24 h) |
IFN-γ in HCVcase (48 h) |
IFN-γ in malignant HCV case (48 h) |
TNF –α in HCVcase (48 h) |
TNF –α in malignant HCV case (48 h) |
|
|
Control |
51.3±8.2 |
80.7±9.5 |
33.6±2.7 |
52.1±7.4 |
29.9±5.5 |
44.7±6.1 |
35.5±4.1 |
35.5±4.1 |
|
Methanol ext. |
35.5±7.8a |
64.3±10.2a |
27.3±4.0a |
41.2±6.9a |
24.9±9.1a |
32.3±10.0a |
14.9±1.7a |
28.8±2.8 a |
|
Chloroform ext. |
29.2±9.4a |
57.0±15.5a |
21.5±.1.8a |
33.2±5.3a |
16.6±1.3a,b |
31.5±8.2a |
13.1±.1.4a,b |
20.8±2.3a,b |
|
Butanol ext. |
32.0±6.8a |
69.5±3.87a |
25.4±9.4a |
39.7±6.0a |
21.0±6.3a,b |
35.8±6.0a |
15.2±1.2a |
24.3±2.3a |
ap<0.01 significant differences decrease as compared with control
bp<0.01 significant differences decrease as compared with Methanol ext.
Table3: Effect of different extracts of Ulmus parvifolia on the cytokines of the human ascites fluid NO after 24 and 48 hours
|
NO in HCV case (24 h) |
NO in malignant HCV case (24 h) |
NO in HCV case (48 h) |
NO in malignant HCV case (48 h) |
|
|
Control |
42.5±7.8 |
45.5±3.9 |
33.5±3.5 |
36.2±5.1 |
|
Methanol ext.
|
35.8±1.9a
|
37.8±4.8a |
30.3±2.6a |
32.4±2.7a |
|
Chloroform ext. |
28.1±2.8a |
29.1±3.5a,b |
24.9±1.5a |
26.5±2.1a,b |
|
Butanol ext. |
30.0±2.7a |
36.4±5.2a |
24.3±2.9a |
29.5±3.9a |
ap<0.01 significant differences decrease as compared with control
bp<0.01 significant differences decrease as compared with Methanol ext.
Table4: Effect of compounds [1-5] on IFN-γ of the human ascites fluid after 24 and 48 hours
|
IFN-γ in HCV case (24 h) |
IFN-γ in HCV case (48 h) |
IFN-γ in malignant HCV case (24 h) |
IFN-γ in malignant HCV case (48 h) |
|
|
Control |
51.3±8.2 |
29.9±5.5 |
80.7±9.5 |
44.7±6.1 |
|
Comp.1 |
39.8±1.5a |
22.4±1.9a |
69.8±1.8a |
35.8±0.58a |
|
Comp.2 |
26.8±2.32a,b |
21.6±1.25a |
54.8±2.83a,b,d |
29.6±0.43a,b |
|
Comp.3 |
31.0±2.89a,b |
20.2±2.7a |
63.3±2.2a |
30.8±0.50a |
|
Comp.4 |
38.5±3.3 a,d |
21.3±2.2a |
64.8±3.2a,c,d |
34.8±2.89a,c |
|
Comp.5 |
23.6±3.6a,b |
19.5±3.1a |
59.9±2.9a,b |
30.0±1.8a |
|
MeOH ext. |
35.5±7.8a |
24.9±9.1a |
64.3±10.2a |
28.8±2.8a |
ap<0.01Significant differences decrease as compared with control
bp<0.01Significant differences decrease comp. 2, comp. 5 and comp. 3 as compared with comp. 1
cp<0.01Significant differences increase comp. 4 as compared with comp. 5
dp<0.05 Significant differences decrease comp. 4 as compared with comp. 2
Table5: Effect of compounds [1-5] on TNF –α of the human ascites fluid after 24 and 48 hours.
|
TNF–α in HCV case (24 h) |
TNF –α in HCV case (48 h) |
TNF–α in HCV case (24 h) |
TNF –α in malignant HCV case (48 h) |
|
|
Control |
33.69±4.67 |
21.9±2.67 |
52.1±7.38 |
35.5±4.1 |
|
Comp.1 |
26.9±0.54a |
17.2±0.31a |
41.68±0.43a |
32.3±0.55a |
|
Comp.2 |
19.5±0.43a,b |
14.1±0.16a |
24.9±0.16a |
16.6±0.16a |
|
Comp.3 |
22.4±0.23a |
13.8±0.14a |
35.8±0.14a |
21.1±0.5a |
|
Comp.4 |
25.2±0.16a |
15.6±0.15a |
30.3±.30a |
24.9±0.6a |
|
Comp.5 |
18.1±0.07a,b,c |
12.3±0.17a |
27.3±0.13a |
21.2±.0.67a |
|
MeOH ext. |
27.3±4.0a,e |
14.9±1.7a |
41.2±6.9a |
28.8±2.8 a |
ap<0.01 Significant differences decrease as compared with control
bp<0.01 Significant differences decreaseComp.2, Comp.3 and Comp.5 as compared with Comp.1
cp<0.01 Significant differences decrease as compared with Comp.4
ep<0.01 Significant differences increase as compared with Comp.5
Table6: Effect of compounds [1-5] on NO of the human ascites fluid after 24 and 48 hours
|
NO in HCV case (24 h) |
NO in HCV case (48 h) |
NO in malignant HCV case (24 h) |
NO in malignant HCV case (48 h) |
|
|
Control |
42.5±7.8 |
33.5±3.5 |
45.5±3.9 |
36.2±5.1 |
|
Comp.1 |
36.3±0.97a |
27.5±1.2 a |
34.48±0.22a |
26.3±0.22a |
|
Comp.2 |
27.5±1.74a |
21.8±2.6 a |
24.9±0.50a |
25.0±0.71a |
|
Comp.3 |
28.4±0.82a |
23.4±1.46 a |
27.8±0.12a |
24.3±0.23a |
|
Comp.4 |
31.6±1.66a |
25.5±0.48 a |
40.9±0.75a |
24.16±1.47a |
|
Comp.5 |
25.4±0.62a |
21.78±3.36 a |
27.1±0.24a |
21.5±1.60a |
| MeOH ext. |
35.8±1.9a
|
30.3±2.6a |
37.8±4.8a |
32.4±2.7a |
ap<0.01Significant differences decrease as compared with control
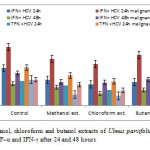 |
Figure1: Effect of methanol, chloroform and butanol extracts of Ulmus parvifolia on the cytokines of the human ascites fluid TNF–α and IFN-γ after 24 and 48 hours Click here to View figure |
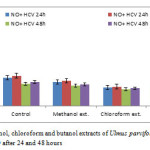 |
Figure2: Effect of methanol, chloroform and butanol extracts of Ulmus parvifolia on the cytokines of the human ascites fluid NO after 24 and 48 hours Click here to View figure |
![Fig. 3: Effect of compounds [1-5] on IFN-γ of the human ascites fluid after 24 and 48 hours](http://www.orientjchem.org/wp-content/uploads/2015/07/Vol31_No3_Anti_Mana_Fig3-150x150.jpg) |
Figure3: Effect of compounds [1-5] on IFN-γ of the human ascites fluid after 24 and 48 hours Click here to View figure |
![Fig. 4: Effect of compounds [1-5] on IFN-γ of the human ascites fluid HCV with cancer after 24 and 48 hours](http://www.orientjchem.org/wp-content/uploads/2015/07/Vol31_No3_Anti_Mana_Fig4-150x150.jpg) |
Figire4: Effect of compounds [1-5] on IFN-γ of the human ascites fluid HCV with cancer after 24 and 48 hours Click here to View figure |
![Fig. 5: Effect of compounds [1-5] on TNF–α of the human ascites fluid after 24 and 48 hours](http://www.orientjchem.org/wp-content/uploads/2015/07/Vol31_No3_Anti_Mana_Fig5-150x150.jpg) |
Figure5: Effect of compounds [1-5] on TNF–α of the human ascites fluid after 24 and 48 hours Click here to View figure |
![Fig. 6: Effect of compounds [1-5] on TNF–α of the human ascites fluid HCV with cancer after 24 and 48 hours](http://www.orientjchem.org/wp-content/uploads/2015/07/Vol31_No3_Anti_Mana_Fig6-150x150.jpg) |
Figure6: Effect of compounds [1-5] on TNF–α of the human ascites fluid HCV with cancer after 24 and 48 hours Click here to View figure |
![Fig. 7: Effect of compounds [1-5] on NO of the human ascites fluid after 24 and48 hours](http://www.orientjchem.org/wp-content/uploads/2015/07/Vol31_No3_Anti_Mana_Fig7-150x150.jpg) |
Figure7: Effect of compounds [1-5] on NO of the human ascites fluid after 24 and48 hours Click here to View figure |
![Fig. 8: Effect of compounds [1-5] on NO of the human ascites fluid HCV with cancer after 24 and 48 hours](http://www.orientjchem.org/wp-content/uploads/2015/07/Vol31_No3_Anti_Mana_Fig8-150x150.jpg) |
Figure8: Effect of compounds [1-5] on NO of the human ascites fluid HCV with cancer after 24 and 48 hours Click here to View figure |
Discussion
Herbal medication as a complementary treatment method is widely used in many variety of diseases, several plant extracts and/or isolated compounds exhibited anticancer, antiviral, anti-inflammatory and other biological activities.
Chromatographic fractionation of butanol extract of U. parvifolia lead to isolation of four compounds identified as Hederagenin 3-O-ß-D-glucopyranoside (1) (22,23), Kaempferol 3-O-ß-D-glucopyranosyl-(1→2)-α-L-rhamnopy-ranosyl-(1→2)-ß-D-glucopyranosyl-7-O-ß-D-glucopyranoside (2) (24), 24-hydroxy-24-methylcycloartanol trans-ferulate (3) (25) and lupeolcaffeate (4) (26). Compounds 1 & 5 were isolated from chloroform extract. Compound 5 was previously isolated from Ulmus davidiana and known as Ulmicin D (27). All compounds were isolated for the first time from U. parvifolia. Identification of the isolated compounds based on chemical and spectroscopic analysis as 1H, 13CNMR (table 1) and mass spectra.
Concerning a search for plant extracts and compounds derived from plants with efficacy as modulators of inflammation, antiviral and anticancer agents, three extracts from Ulmus parvifolia, and five isolated compounds were investigated for their anticancer and antiviral effect on three cytokines of the human ascites fluid, Interferons-gamma, TNF-alpha and NO. The cytokine interferon-γ is one of the major cytokines produced by Th1 cells in response to viral infections and it is a potent activator of other T & B lymphocytes. TNF-α is produced by activated macrophages, fibroblast, and many different cells and well characterized as one of the important defense molecules of body with potent pro-inflammatory effects (28).
Treatment with extracts and isolated compounds showed significantly decrease in the immunological parameters as compared with control (p< 0.01) after 24 and 48 hours [Tables 2-6]. The results in this study showed significant decrease in the three cytokines IFN-γ, TNF-α and NO, which indicates that the high curative effect of both plant extracts and the isolated compounds. These significant decrease results were due to the regulation of induced apoptotic cell death and inhibition of inflammation and tumorigenesis and viral replication (29).
Tables [2 & 3] showed (mean + SD) the results of the effect of the three extracts on the three cytokines after 24 & 48 hours, chloroform extract showed significant decrease as compared with methanol and butanol extracts, and butanol extract showed significant decrease compared to control. This activity may be due to triterpenoids (30) and phenolic contents of the plant (31). The results showed high response to the extracts in HCV cases than that of malignant HCV cases, it also showed that more exposure to drugs from 24 to 48 hours give more significant decrease and more curative effect.
Tables [4-6] showed (mean + SD) the results of the effect of the isolated compounds (1-5) on the three cytokine in the same case after 24 and 48 hours. All the isolated compounds showed significant decrease as compared with control (p<0.01). Compound 5 (Ulmicin D) showed significant decrease (higher effect) compared with control and with original methanol extract (p<0.01) in HCV case on the three cytokines of the human ascites fluid after 24 and 48 hours, but compound 2 (Kaempferol 3-O-ß-D-glucopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-ß-D-glucopyranosyl-7-O-ß-D-glucopyra-noside) showed higher effect in malignant HCV case. These results may be due to excessive production of NO inmalignant HCV cases, since NO is induced in macrophages that have antitumor activity through the inhibition of DNA synthesis in tumor cells, which may also induce apoptosis (32).
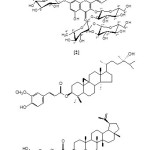 |
Scheme 1 Click here to View scheme |
Conclusions
Methanol, chloroform and butanol extracts of Ulmus pravifolia were evaluated for anticancer and antiviral activity; they were tested on three cytokines of the human ascites fluid, TNF-alpha, Interferons-gamma and NO. Both chlorophorm and butanol extracts showed a significant curative effect. Five compounds were isolated from the plant and identified as Hederagenin 3-O-ß-D-glucopyranoside, Kaempferol 3-O–ß-D-glucopyranosyl-(1→2)-α-L-Rhamnopyranosyl-(1→2)-O-ß-D-gluco-pyranosyl-7-O–ß-D-glucop-yranoside, 24-hydroxy-24-methylcycloartanol trans-ferulate, Lupeol caffeate and Ulmicin D. All the isolated compounds showed significant activity as compared with control. Compound 5 (Ulmicin D) showed higher effect compared to original methanol extract in HCV case on IFN-γ of the human ascites fluid after 24 and 48 hours, but compound 2 (Kaempferol 3-O–ß-D-glucopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-O-ß-D-glucopyranosyl-7-O–ß-D-glucop-yranoside) showed higher effect in malignant HCV case.
References
- Madhuri, S.; Pandey, G. Some anticancer medicinal plants of foreign origin. Curr. Sci. 2009, 96, 779-783
- Imawari, M. Liver Cancer-Prevention and Early Diagnosis. Clinical Medicine: Cancer, 2002, 45, 130–133
- Hamed, M. M. Volatile oils from Pelargonium zonale and its cytotoxic effect on tumor cells. Bull. Fac. Pharm. Cairo Univ. 2007, 45, (3), 297-300
- Rosangkima, G.; Prasad, S. Antitumour activity of some plants from Meghalaya and Mizoram against murine ascites Dolton’s lymphoma. Indian J. Exp. Biol. 2004, 42, 981–988
- Sirko, A.; Vaněk, T.; Góra-Sochacka, A. ; Redkiewicz, P. Recombinant cytokines from plants. J. Mol. Sci. 2011, 12, 3536-3552
- Heywood, V. H. Flowering Plants of the World. Batsford, London. 1993, PP 336
- Suh, S.; Yun,W.; Kim, K.; Jin,U.; Kim, J.; Kim, M.; Kwon, D.; Kim Y.C. Stimulative effects of Ulmus davidiana Planch (Ulmaceae) on osteoblastic MC3T3-E1 cells. J. Ethnopharmacol. 2007, 109, 480-5
- Paschke, D.; Abarzua, S.; Schlichting, A.; Richter, D.; Leinweber, P.; Briese, V. Inhibitory effects of bark extracts from Ulmus laevis on endometrial carcinoma: an in-vitro study. Eur J Cancer Prev. 2009, 18 (2): 162-168
- Song, I.; Kim, K.; Suh, S.; Kim, M.; Kwon,D.; Kim, S.; Kim C. Anti-inflammatory effect of Ulmus davidiana Planch (Ulmaceae) on collagen-induced inflammation in rats. Environ Toxicol. Pharm. 2007, 23, 102–110
- Hwa, K. G.; Ju, K. Y.; Jin, O. Y.; Ryeol, Y. Y. Polysaccharides containing peptides originated from elm and protection thereof. Repub. Korean Kongkae Taeho Kongbo KR. 2001, 68, 625
- Jung, H.; Jeon, H.; Lim, E.; Ahn, E.; Song, Y. S.; Lee, S.; Shin, K. H.; Lim, C.; Park, E. Anti- angiogenic activity of the methanol extract and its fractions of Ulmus davidiana var. japonica. J. Ethnopharmacol. 2007, 112, 406-409
- Overeem, J. C.; elgersma, D. M. Accumulation of masonones E and F in Ulmus hollandica infected with Ceratocystisulmi. Phyochemistry, 1970, 9, 1949-1952
- Giannasi, D. E. Generic relationship in the Ulmaceae based on flavonoid chemistry. Taxon, 1978, 27, 331-334
- Heimler, D.; Mittempergher, L.; Buzzini, P.; Boddi, V. Quantitative HPTLC separation of flavonoid glycosides in the taxonomy of elm (Ulmus spp.). Chromatographia, 1990, 29, 1-2
- Rowe, J. W.; Anthony, C. Extractives in eastern hardwoods- A Review. 1979, PP 45-47
- Hassanein, H. I.; El-ahwany, E. G.; Salah, F.M.; Hammam, O. A.; Refai L.;Hamed M. Extracts of Five Medicinal Herbs Induced Cytotoxicity in Both Hepatoma and Myeloma Cell Lines. J. Cancer Sci. Ther. 2011, 3, 239-243
- Köhler, G.; Milstein, C. Continuous cultures of fused cell secreting antibody of predefined specificity. Nature, 1975, 256, 495-7
- Hamed, M. M.; El-Amin, S. M.; Refahy, L. A.; Saad, A. M.; Mansour, W. A.; Abu Taleb, H. M.; El-Ansary, M. Isolation of some chemical constituents from licorice and their evaluation as anticancer and antiviral agents. Pharmacology online, 2013, 3, 95-109
- Tracey, W. R.; Tse, J.; Carter, G. Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: pharmacological evaluation of nitric oxide synthase inhibitors. J. Pharmacol. Exp. Ther. 1995, 272, 1011-5
- De Myer, E.; De Mayer G.J; Interferom g. Curr. Opinion. Immunology, 1992, 4, 321-332
- Damas, P.; Peuter, A.; Gysen, P.; Demonty, J.; Lamy, M.; Franchimont, P. Tumor Necrosis Factor and interleukin 1 serum levels during severe sepsis in humans. Crit. Care. Med. 1989, 17, 975-983
- Jhoo, J.; Sang, S.; He, K.; Cheng, X.; Zhu, N.; Stark, R.E.; Zheng, Q.Y.; Rosen, R.T.; Ho, C. Characterization of the triterpene saponins of the roots and rhizomes of blue cohosh (caulophyllum thalictroides). J. Agr. Food Chem. 2001, 49, 5969-5974
- Yin, J.Y.; Meng, Q.; Wong, E.S. Septemloside III: A nonasaccharide saponin from the bark of kalopanax septemlobus (thumb.) koidz. Chinese Chem. Lett. 2005, 16, 53-56
- Gohar, A.; Moatooq, G. T.; Niwa, M. Two flavonoid glycoside from chenopodium murale. Phytochemistry, 2000, 53, 299-303
- Fang, N.; Yu, S.; Badger, T. M. Characterization of triterpene alcohol and sterol ferulates in rice bran using LC-MS/MS. J. Agr. Food Chem. 2003, 51, 3260-7
- Alvarenga, N.; Ferro, E. A. A new lupane caffeoyl ester from Hippocratea volubilis. Fitoterapia, 2000, 71, 719-721
- Lee, M. k.; Kim, Y. C. Five Novel Neuroprotective Triterpene Esters of Ulmus davidiana var. japonica. J. Nat. Prod. 2001, 64, 328-331
- Rojas, V.; Morales-Lange, B.; Guzmán, F.; Gallardo, J. A.; Mercado, L. Immunological strategy for detecting the pro-inflammatory cytokine TNF-alpha in salmonids. Electron. J. Biotechnol. 2012, 15, 1-7
- Wang, Y.; Lo, G.; Lai, K.; Cheng, J.; Lin, C.; Hsu, P. Increased Serum Concentrations of Tumor Necrosis Factor-α Are Associated with Disease Progression and Malnutrition in Hepatocellular Carcinoma. J. Chin. Med. Assoc. 2003, 66, 592-597
- Wang, D.; Xia, M.; Cui,Z. New Triterpenoids Isolated from the Root Bark of Ulmus pumila L. Chem. Pharm. Bull. 2006, 54, 775-778
- Kwon, J.; Kim, S.; Park K.; Lee M. Antioxidative and anti-inflammatory effects of phenolic compounds from the roots of Ulmus macrocarpa. Arch. Pharm. Res. 2011, 34, 1459-1466
- Wolf, H.; Haeckel, C.; Roessner, A. Inducible nitric oxide synthase expression in human urinary bladder cancer. Virchows Arch. 2000, 437, 662-666

This work is licensed under a Creative Commons Attribution 4.0 International License.









