13C and 17O NMR of Mono-Nuclear Carbonyls: A DFT Study
Seema Sharma1, Subhash Chander2, M. L. Sehgal3, Irshad Ahmad4*
1Department of Chemistry, Giani Zail Singh Punjab Technical Campus, Bathinda- 151001,India. 2P.T.U., Jalandhar-144001, India. 3Department of Chemistry, D.A.V. College, Jalandhar-144008, India. 4Department of Biochemistry, Faculty of Life Sciences, A. M.U, Aligarh -202002,India. Correspondence Author Email: irshadahmad.bio@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310320
Article Received on :
Article Accepted on :
Article Published : 20 Aug 2015
DFT implemented in ADF 2012.01 was applied to11 mono-nuclear carbonyls after their optimization to ascertain the stereo chemical and magnetic equivalence of CO groups and calculation of Effective Spin Hamiltonian(H Spin) values of the metals and the carbon atoms after obtaining NMR parameters like Chemical Shifts (δ M n+,δ13C,δ17O),total NMR shielding tensors(σ Mn+,σ13C,σ17O) consisting of 2 diamagnetic and 4 paramagnetic terms along with k and j parameters of constituents. This NMR study corroborated well the hitherto, already, reported IR/ Raman results to lend credence to the π - acid character of carbonyls.
KEYWORDS:Chemical Shift; Shielding Tensor; Paramagnetic Tensor; Effective Spin Hamiltonian; Magnetic Equivalence
Download this article as:| Copy the following to cite this article: Sharma S, Chander S, Sehgal M. L, Ahmad I. 13C and 17O NMR of Mono-Nuclear Carbonyls: A DFT Study. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Sharma S, Chander S, Sehgal M. L, Ahmad I. 13C and 17O NMR of Mono-Nuclear Carbonyls: A DFT Study. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10360 |
Introduction
DFT was, earlier, applied by Schreckenbach et al [1-15] to study 13C and 17O NMR spectra of some mononuclear transition metal carbonyls. But the present work would include the study of a number of NMR parameters such as Chemical Shifts of metal, the carbon and the oxygen species (δ M,δ13C, δ 17O), their Total NMR Shielding Tensors(σ M, σ 13C,σ17O ) along with two diamagnetic contributions [diamagnetic core tensor{a} and diamagnetic valence tensor{b}] and four paramagnetic contributions [paramagnetic (b^) tensor{c}, paramagnetic (u^) tensor{d}, paramagnetic(s^) tensor{e} and paramagnetic gauge tensor(f). Algebraic sum of these 6 contributions was equal to their σ M, σ 13C, σ 17O respectively. The parameters like Fermi-contact (k) [1019 kg m-2 s-2 A-2], spin-spin coupling (j) [p pm] were also obtained.
While the discussion on NMR of transition metal complexes [16, 17] encircled around ligand field theory [18], in the late 70s, a number of review articles were collected[19] on small molecules. Debrochere (1978) published a 100 page review containing 289 references [20] .But till then, no calculations on nuclear shielding and spin-spin coupling parameters were carried out. H F approach given by Nakatsuzi [21] presented a paper on the calculation of the above named parameters of the complexes was found lacking in high oxidation states d10 systems[22]. In 80s, the NMR shielding codes based on HFSor X α method were developed. It was afterward called DFT [23-25]. In 1993, Kohn-Sham DFT [26, 27] employed IGLO [26-27] method to calculate nuclear shielding. Also, LORG approach [27] which was improved upon by GIAODFT [28] and CSGTmethods [29] was employed. Spin-spin coupling constant of complexes was first of all calculated by Malkin et al [30]. Dicken and Zieglar [31] calculated FC term [32] in1996. Later, SD term [33, 34] was included in spin- spin coupling values.
The 11 mono-nuclear carbonyls were included in this study : [M (CO)6](M=Cr, Mo, W), [V(CO)6]1- ,[M(CO)6]1+ (M=Mn , Re), [M(CO5)] (M=Fe, Ru Os),[Co(CO)4]1- and [Ni(CO)4] . All these carbonyls obeyed the 18 electron rule.
Need of the Study
Three points necessitated this study as:
- No computational studies were reported on the magnetic equivalence of COs. Only their spatial displacements/ stereo chemical equivalences were studied.
- An important NMR parameter- Effective Spin Hamiltonian (H Spin) [17] which determines the energy of an NMR transition had, never, been calculated by DFT.
- DFT had, hardly, been applied to NMR in ascertaining the pi-acid character of metal carbonyls though IR/ Raman techniques had abundantly been exploited.
Methodology
[3, 13, 32, 35, 36, 37]
ADF software was installed on Windows XP platform as “ADF jobs”. A new directory was created using “File menu” of ADF jobs.
After optimization of the carbonyl compound, different commands were filled into the software to obtain NMR and IR/Raman parameters as follows:
NMR Parameters
[36, 37]
The software was run by filling in certain commands like Single Point, LDA, Default, None, Collinear, Nysom using DZ or TPZ Basis sets. The Unrestricted command was left blank. Then “NMR Program” was run in three steps.
- The Shielding Constants of the constituents (σM ,σ 13C,σ 17O) were obtained from the “NMR Program” by clicking on numbers of the species and printing them along with “Isotropic Shielding Constants” and “Full Shielding Constants”. The Chemical Shifts (δ M, δ13C, δ17O) were obtained from their NMR spectra.
- k and j values of constituents were obtained from the same program by using a new Input File and printing numbers of Perturbing and Responding nuclei.
- σ 13C, σ17O , δ 13C, δ17O,k and j of C and O of uncoordinated CO (g) were obtained by repeating the above mentioned two steps with reference values σ 13C (-34.44) and σ17 O(-129.53).
IR and Raman Parameters
After Optimization, the software is run with Frequencies and Raman full to obtain values of frequencies of all the (3n-6) Fundamental vibration bands.
Results
[I] Table: 1 contained Acronyms and their expanded forms. Tables: 2-3 gave the optimization[38,39]and thermal parameters of the carbonyls respectively.Table:4 contained eight NMR parameters: three shielding constants(σM,σ13C,σ17O ),three Chemical Shifts(δ M ,δ13C, δ 17O) and two Coordination Shifts(Δ δ 13C , Δ δ17O ) .Six contributions consisting of two diamagnetic and four paramagnetic terms of three parameters (α M, α13C, α17O) were given in Tables:5-7[{a},{b}.{c},{d},{e}.{f}].Total values of two diamagnetic and four paramagnetic contributions in σ M, σ13C and σ 17O were given in Table: 8. Table:9 represented Spatial and Magnetic Equivalence of CO groups. Table: 10 contained k and j parameters given by software and the H spin values as calculated from j values.
Table 1: Acronyms and Their Expanded Forms
| DFT | Density Functional Theory |
| ADF | Amsterdam Density Functional |
| ZORA | Zeroth- Order Regular Approximation |
| LDA | Local Density Approximation |
| DZ/TPZ | Double Zeta/ Triple Zeta |
| GGABP | Generalized Gradient Approximation |
| GGABP | Generalized Gradient Approximation Becke Perdew |
| Nysom | Normalized or True |
| H F | Hartree- Fock |
| HFS | Hartree-Fock-Dickson-Slater |
| IGLO | Independent or Individual Gauge of Localized Orbitals |
| LORG | Localized Orbitals Resonance Gauge |
| GIAO | Gauge Including Atomic Orbitals |
| CSGT | Continuous Set of Gauge Transformation |
| SD/ FC | Spin-dipole/ Fermi-contact |
Table 2: Optimization Parameters of Mononuclear Carbonyls
| Carbonyl (≈0.0D) | Point group | Total bonding Energy | Total Energy: X c (LDA) k J mole-1 | Nucleus | I |
| [V(CO)6 ]1– | Oh | -9885.20 | -309197.58(-289776.63, -19420.95) | 51V | 3.5 |
| [ Cr(CO)6] | -do- | -9749.83 | -317868.84(-298129.57,-19739.27) | 53Cr | 1.5 |
| [ Mo(CO)6] | -do- | -9761.76 | -504440.84(-479759.83, -24681.02) | 95Mo | 2.5 |
| [W(CO)6] | -do- | -9991.96 | -981514.64(-947053.82, -34460.82) | 183W | 0.5 |
| [Mn(CO)6]1+ | -do- | -8833.60 | -326285.10(-306302.80,-19982.30) | 55Mn | 2.5 |
| [Re(CO)6]1+ | -do- | -9230.86 | -999049.56(-964269.91, -34779.65) | 185Re | 2.5 |
| [Co(CO)4]1- | Td | -6757.34 | -279321.74(-263582.63, -15739.11) | 59Co | 0.5 |
| [Ni(CO)4] | -do- | -6232.74 | -289903.76(-273830.40, -16073.36) | 61Ni | 2.5 |
| [Fe(CO)5] | D3h | -8029.69 | 302622.68(-284764.22, -17858.46) | 57Fe | 1.5 |
| [Ru(CO)5] | -do- | -7986.29 | -496080.13(-473217.51, -22862.62) | 101Ru | 3.5 |
| [Os(CO)5] | -do- | -8364.94 | -984410.88(-951721.35, -32689.52) | 187Os | 1.5 |
*X c is made up of LDA and GGA components; which further contain Exchange and Correlation
parts. Bonding energy is computed as an energy difference between the molecule and fragments. GGA is zero here.
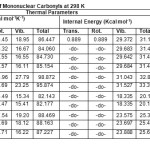 |
Table 3: Thermal Parameters of Mononuclear Carbonyls at 298 K Click here to View table |
![Table: 4. σ, δ and Δ δ [ppm] values of M, C and O in Mononuclear Carbonyls](http://www.orientjchem.org/wp-content/uploads/2015/08/Vol31_No3_13C_Seem_T4-150x150.jpg) |
Table : 4. σ, δ and Δ δ [ppm] values of M, C and O in Mononuclear Carbonyls Click here to View table |
Table 5: Shielding Constants [p pm] of M, Diamagnetic and Paramagnetic Contributions
| Carbonyl | σM( MCO) | Diamagnetic Contributions{a} {b} | Paramagnetic Contributions{c} {d} {e} {f} |
| [V(CO)6 ]1- | -141.68 | 1660.804,51.650 | -250.309, -2096.566,493.643,-0.905 |
| [Cr(CO)6 ] | -1428.92 | 0.000 , 1821.872 | 0.000, -3512.409, 262.589 , -0.975 |
| [Mo(CO)6] | 1328.44 | 3936.345,60.030 | -745.997,-2900.566,979.771,-1.144 |
| [W(CO)6] | 4717.10 | 8530.161,198.232 | 680.920,-3665.371,-1027.367, 0.524 |
| [Mn(CO)6]1+ | -4718.18 | 1825.324,101.135 | -229.740,-6590.544,717.941,-2.301 |
| [Re(CO)6]1+ | 3296.33 | 8670.042,218.625 | 686.109,-5466.369, -812.461,0.385 |
| [Co(CO)4]1- | -3771.98 | 1989.201,171.689 | -117.023,-6423.724, 607.811,0.071 |
| [Ni(CO)4] | -2050.46 | 2070.943,208.080 | -132.181,-4552.960, 355.809,-0.147 |
| [Fe(CO)5] | -5117.03 | 1907.276,134.245 | -170.891,-7686.421,699.538,-0.779 |
| [Ru(CO)5] | -993.92 | 4161.943,103.726 | -574.583, -5456.581,771.062, 0.513 |
| [Os(CO)5] | 2061.72 | 8814.709,246.542 | 569.979, -6992.715, -578.172, 1.377 |
Table 6: Shielding Constants [p pm] of 13C, Diamagnetic and Paramagnetic Contributions
| Carbonyl | σ13C(MCO) | Diamagnetic Contributions{a} {b} | Para magnetic Contributions{c} {d} {e} {f} |
| [V(CO)6]1- | -25.45 | 199.233,47.944 | 0.028 , -313.186, 39.704, 0.785 |
| [Cr(CO)6] | -20.90 | 0.000 , 253.438 | 0.000 ,-301.057, 26.316 ,0.413 |
| [Mo(CO)6 ] | -12.02 | 199.236, 50.566 | 0.080 ,-296.744, 34.131, 0.716 |
| [W(CO)6] | -8.23 | 199.232 ,50.259 | -0.018 ,-295.857,37.915, 0.241 |
| [Mn(CO)6]1+ | -20.05 | 199.233,50.516 | -0.014,-304.470, 34.677,-0.001 |
| [Re(CO)6 ]1+ | -2.900 | 199.232 ,51.670 | -0.030,-288.485,34.817,-0.116 |
| [Co(CO)4]1- | -19.22 | 199.233 ,50.726 | 0.037,-309.605, 40.207,0.178 |
| [Ni(CO)4] | 1.12 | 199.233 ,51.411 | 0.031 ,-285.869,36.213,0.105 |
| [Fe(CO)5] | -24.80(e)-56.30(a) | 199.233 ,50.701199.233 ,51.687 | 0.007,-312.523, 37.832, -0.0600.031, -339.899,32.266,0.384 |
| [Ru(CO)5] | -18.50(e)-36.17(a) | 199.234 ,51.838199.234 ,53.295 | 0.045, -299.689, 29.641, 0.4140.096 , -316.614,27.040,0.775 |
| [Os(CO)5] | -18.79(e)-30.6 (a) | 199.233, 53.412199.233 ,51.367 | -0.001,-313.307,29.599, 0.4460.008 ,-304.234, 34.814,0.020 |
Table 7: Shielding Constants [p pm] of 17O, Diamagnetic and Paramagnetic Contributions
| Carbonyl | σ 17O (MCO) | Diamagnetic Contributions{a} {b} | Para magnetic Contributions{c} {d} {e} {f} |
| [V(CO)6 ]1- | -45.97 | 269.471 ,133.899 | -0.082, -392.033, -56.213 ,-1.060 |
| [Cr(CO)6 ] | -74.97 | 0.000 , 397.850 | 0.000 ,-447.416, -24.582, -0.901 |
| [Mo(CO)6] | -58.15 | 269.471 ,130.426 | 0.006 , -416.371, -41.090 ,-0.597 |
| [W(CO)6] | -51.35 | 269.471,130.294 | -0.003, -413.992, -36.338, -0.809 |
| [Mn(CO)6]1+ | -123.50 | 269.471, 127.036 | 0.019 , -490.879, -28.269, -0.862 |
| [Re(CO)6]1+ | -83.06 | 269.471,126.889 | 0.037, -454.952, -23.710 , -0.760 |
| [Co(CO)4]1- | -34.20 | 269.472 ,134.157 | 0.030 , -385.614, -52.149 ,-0.091 |
| [Ni(CO)4] | -42.90 | 269.472 , 130.513 | 0.048. -402.968, -39.838, -0.125 |
| [Fe(CO)5] | -77.5 (e)-162.77(a) | 269.472 , 130.303269.471 ,129.490 | 0.016 , -435.846, -40.978, -0.4170.016 , -533.743, -27.475, -0.532 |
| [Ru (CO)5] | -82.00 (e)-117.25(a) | 269.472 , 130.243269.471, 129.274 | 0.007 ,-435.040, -46.462, -0.223-0.007 , -489.099, -26.478,-0.406 |
| [Os(CO)5] | -85.00(e)-104.50(a) | 269.472 , 130.204269.471, 129.192 | 0.018 , -442.812, -41.543 ,-0.3400.001, -481.733, -20.938, -0.479 |
![Table 8: Total Diamagnetic, Paramagnetic contributions in σ M, σ13C and σ 17O [p pm]](http://www.orientjchem.org/wp-content/uploads/2015/08/Vol31_No3_13C_Seem_T8-150x150.jpg) |
Table 8: Total Diamagnetic, Paramagnetic contributions in σ M, σ13C and σ 17O [p pm] Click here to View table |
Table 9: Spatial and Magnetic Equivalence of COs in Mononuclear Carbonyls
| Carbonyl | SpatiallyEquivalent COs | Magnetically Equivalent COs | Types of σ 13C & σ 17O | No. of Spatially Different COs |
| *[V(CO)6]1- | All | All | One | Same type of six COs |
| [Cr(CO)6] | -do- | 3 types;2 in each type | -do- | -do- |
| [Mo(CO)6] | -do- | -do- | -do- | -do- |
| [W(CO)6] | -do- | -do- | -do- | -do- |
| [Mn(CO)6]1 | -do- | -do- | -do- | -do- |
| [Re(CO)6]1+ | -do- | -do- | -do- | -do- |
| *[Co(CO)4]1- | -do- | All | One | Same type of four COs |
| *[Ni(CO)4] | -do- | -do- | -do- | -do- |
| [Fe(CO)5] | 2 types;3(e) and 2(a) | 2 types ;3(e) and 2(a) | Two | 2 types ;3(e) and 2(a) |
| [Ru(CO)5] | -do- | -do- | -do- | -do- |
| [Os(CO)5] | -do- | -do- | -do- | -do- |
*All COs are both spatially and magnetically equivalent
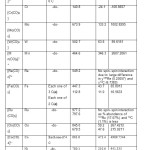 |
Table 10: k, j and H spin values of Nuclei in Mononuclear Carbonyls Click here to View table |
Discussion
The discussion was divided into eight headings as follows:
(A) As stated, the software gave a number of parameters which were further related a number of other parameters as follow:
(i)Sum of 6 contributions was equal to their σ M, σ 13C,, σ 17O[p pm] respectively.
σ M =Sum of 2 diamagnetic and 4 paramagnetic contributions of M |
σ 13C =Sum of 2 diamagnetic and 4 paramagnetic contributions of 13C| [1]
σ 17O =Sum of 2 diamagnetic and 4 paramagnetic contributions of 17O|
(ii)The relation between (σ) and (δ) of carbon was given as:
δ13C=181.1- σ 13C——————————————————————– [2]
(iii)δM and δ17O were numerically equal to σ M and σ 17O but with opposite signs
σ M = – δ M |
| ———————————————— [3]
σ17O= – δ 17O |
(iv)The Coordination Shifts [Δδ13C, Δδ17O] and [σ 13C, σ17O] were related as:
Δ δ13C= α13C (MCO) –(- 34.44) ————————————————— [4]
Δ δ 17O = α17O (MCO) – (-129.53) ————————————————- [5]
[B]Relative spatial displacements of constituting species were reaffirmed from shielding constants of the M, C and O [σM, σ 13C (M CO), σ 17O (MCO)] simply by the fact that the spatially equivalent species should have same values of shielding constants along with their constituting two diamagnetic and four paramagnetic terms respectively. All the 13C and 17O nuclei in each one of the four or six CO groups in Td or Oh possessed the same values of σ, δ and also the six contributing diamagnetic and paramagnetic quantities respectively. Therefore, all the four or six CO ligands were in the same spatial displacement, i.e. stereochemically equivalent around their respective central metal ion.It would be easy to conclude that that more the value σ13C,the lesser should be the value of Chemical shift (δ 13C) and more would be the value of Coordination Shift (Δ δ13 C) for a given stereochemistry of the carbonyls as illustrated below:
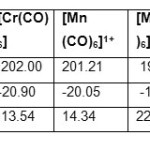 |
Table 11 Click here to View table |
[C] In D3h stereochemistry, each one of the three 13C and 17O had the same values of σ13C, δ13C,σ17O and δ17O along with their six contributing terms respectively; meaning thereby that the three COs were stereo chemically equivalents. Each one of the remaining two COs also possessed same values of σ13C, δ13C,σ17O and δ17O along with their contributing terms respectively. But these values were quite different from those of the other three CO s. Thus these two COs were different from the other three COs spatially*.
The values of 8 parameters : σ M, δ M, σ 13C, δ 13C, Δ δ13C, σ 17O, δ 17O and Δ δ17O [ pp m] for the11 mononuclear carbonyls were given in Table:4. The more the value of σ 13C,the lower was the Chemical shift (δ13C) and higher was the Coordination Shift (Δ δ 13C) for any stereochemistry. A positive Δ δ 13C is reported in Oh geometry which confirmsthe back-acceptor nature 0f CO. The difference between σ 17 Ovalues of carbonyls and the σ 17O of CO (g) was noted. This positive** shift in Δ δ17 O also confirmed the transfer
*s p3d hybridization in a trigonal bipyrimidal geometry is supplemented by the prolate dz2 orbital having two opposite major lobes (m=0). A pair of opposite vertices [40] (X–M–X angle =180°) makes trigonal bipyramid a prolate polyhedron. Carbonyls occupying these two vertices have more electron density than the other three lying ^ to these two. **Negative in [Fe (CO) 5].
of some electron density from C to O in carbonyls.
With two different types of spatially equivalent CO groups (e, a), the five coordinate D 3h carbonyls showed two types of σ 13C and σ 17O values. Accordingly, two values of coordination shifts called Δ δ13Ca andΔ δ13Ce were obtained. Relative order of Δ δ13Ce in the five coordinate carbonyls is given as:
[Fe (CO)5]˂ [Ru (CO)5] ≈ [Os (CO)5]
Δ δ13Ca follows the order:
[Fe (CO)5]˂ [Ru (CO)5] ˂ [Os (CO)5]
[D]As shielding constant (σ) of a nucleus was directly related to its electron density, any change in its σ value would serve as an indicator of change in electron density on it. So, if CO were to act as a back acceptor, σ 13C of metal carbonyls should become more than σ 13C of CO (g). Some of the increased electron density on carbon was transmitted to oxygen to cause an increase in electron density on oxygen. So σ 17O would also increase. The NMR results corroborate with results obtained from their IR/Raman parameters in confirming their π – acid character as follows:
π- ligand CO would donate electron density to the metal via a dative σ bond (OC → M) .Simultaneously, there would be a π back donation from the filled d orbitals of metal (OC ←M) to energetically favorable and geometrically suitable vacant π* molecular orbitals of CO. The effect being synergic should cause a decrease in carbon oxygen double character and, thus, a decrease in nCO in carbonyls with respect to CO(g) having nCO= 2143 cm1- was expected if C O was to act as a back pi- acceptor. A comparison of σ 13C, δ 13C, Δ δ 13C and nCO
(cm-1)values of the 6 and 5 coordinate(axial) carbonyls in the following table lead to the conclusion that as the Chemical Shift(δ13C)decreased,nCO(cm1-) would increase[41-48] to decrease the capacity to back accept electron cloud by CO.
| Parameters[ p pm] | [V(CO)6]1- | [Mn (CO)6]1+ | [Re(CO) 6]1+ | |
| δ 13C | 206.50 | 201.21 | 184.01 | |
| σ 13C | -25.45 | -20.05 | -2.9 | |
| Δ δ 13C | 8.99 | 14.34 | 31.54 | |
| nCO | 2020.0 | 2192.0 | 2197.0 | |
| Parameters | [Cr(CO)6] | [Mo(CO)6] | [W(CO)6] | |
| δ 13C | 202.00 | 193.12 | 189.33 | |
| σ 13C | -20.90 | -12.02 | -8.23 | |
| Δ δ 13C | 13.54 | 22.42 | 26.21 | |
| nCO | 2118.7 | 2120.7 | 2126.2 | |
| Parameters( Equatorial) | Fe (CO) 5 | Ru (CO) 5 | Os(CO) 5 | |
| δ 13C | 205.91 | 199.63 | 198.89 | |
| σ 13C | -24.81 | -18.50 | -18.79 | |
| Δ δ 13C | 9.63 | 15.92 | 15.65 | |
| nCO | 2022.0 | 2035.0 | 2036.0 | |
| π acceptor strength in all | Maximum | Less | Least | |
[E](i) Another important element of NMR symmetry was called the “magnetic equivalence” of nuclei .Enantiotopic or homotopic nuclei though possessed the same chemical shift (δ), but might not necessarily be magnetically equivalent. Two magnetically equivalent nuclei would have the same values of σ, δ, k and j with other nuclei of the molecule in addition to having same values among themselves .Coupling between symmetry equivalent and magnetically nonequivalent nuclei would affect the appearance of NMR spectrum while coupling between both the symmetry and magnetically equivalent nuclei had no effect NMR spectra.
[ii] With same σ 13C, δ13C, k and j values, the four COs were both spatially and magnetically equivalent in Td .Again, the six CO groups in [V (CO) 6]1- were both spatially and magnetically equivalent with the same σ 13C, δ 13C, k and j values. But the six COs in the remaining (Oh) mono-nuclear carbonyls were only spatially equivalent with same σ 13C, δ 13C values as they possessed different k and j values. They were of three types. Each type having two CO groups possessed both spatial and magnetic equivalence. The three types of CO pairs had the same set of four parameters respectively between themselves and with remaining four CO groups though the set of four COs show different values from the previous set of two COs.
[iii] The five COs (D3h) were neither spatially nor magnetically equivalent as they did not have the same set of four parameters. They consisted of two sets. The first set with two COs (a) and the second with three COs (e) showed both the spatial and magnetic equivalence among themselves as either type possessed same set of values of four parameters among its own members and also with members of other type of CO groups though the two sets have different values of these parameters.
[F] (i) Spin-spin coupling (j) was field-independent and mutual (j AB = j BA).It was affected by the nature of solvent ; metal−ligand bond distances and was transmitted through bonding electrons with its magnitude falling off rapidly with the increase in number of intervening bonds. Its sign was decided as: “it was positive if energy of A was lower when B had opposite spin as A (α β or β α), and negative if energy of A was lower when B had same spin as A (α α or β β )”.
(ii) The parameter (j) was related to another important NMR called Effective Spin Hamiltonian (H Spin). It was a mathematical expression that would determine the energy of an NMR transition. It term “effective” meant that its solutions reproduced nuclear magnetic energy levels in a molecular system without reference to electrons. In a fictitious absence of surrounding electrons, the shielding constants and indirect spin-spin coupling constants would vanish leaving the NMR spectrum to be determined by Nuclear Zeeman Term and direct dipolar coupling. (H Spin) values of the metal ions and the bonded carbon atoms were related to their j [p pm] values as given below [6] (17).
H Spin =6.023j A B. IA. IB.MHz mol-1 ———————————————- ———- [6]
Spin Hamiltonian [H Spin] values of the metal and the bonded carbon atoms of the eleven carbonyls are calculated by applying [5] (Table: 10).
[G]Individual values of 6 diamagnetic and paramagnetic quantities in σ M,σ 13 C and σ 17O and their sums were given in Tables: 5-8 respectively.
[H] Table: 9 showed spatial displacements of 4, 5 or 6 CO groups around the metal.
Conclusions
The originality and relevance of present work and how it moved the body of scientific knowledge forward would lie in the fact that it reaffirmed the relative spatial displacements of CO groups; classified them according to their spatial and magnetic equivalence; lent credence to π – acid character of carbonyls by corroborating with their IR/Raman studies and hence justified the need of taking up this study.
Acknowledgements
Authors gratefully acknowledge the kind and willing cooperation of Mr. Sunil Chawla [sunil@seascapelearning.com] of ADF (http://www.scm.com).They feel indebted to Mr. S.R. Heer, Chief Engineer (Retd.), North Zone, Doordarshan, New Delhi (India), for his invaluable cooperation in the installation and smooth working of the ADF software.
References
- Ruiz-Morales, Y.; Schreckenbach, G.; Ziegler, T. J. Phys. Chem. 1996 100, 3359−67.
- Schreckenbach, G.; Ziegler, T.; Li, J. Int. J. Quantum Chem. 1995 56, 477−88.
- Schreckenbach, G.; Ziegler, T. J. Phys. Chem. 1995 99, 606-11.
- Li, J.; Schreckenbach, G.; Ziegler, T. Inorg. Chem. 1995 34, 3245−52.
- Li, J.; Schreckenbach, G.; Ziegler, T. J. Am. Chem. Soc. 1995 117, 486−94.
- Li, J.; Schreckenbach, G.; Ziegler, T. J. Phys. Chem., 1994 98, 4838−41.
- Popenova, S.; Mawhinney, R. C.; Schreckenbach, G. Inorg. Chem. 2007 46(10), 3856-64.
- Schreckenbach, G. Inorg. Chem. 2002 41(25), 6560-72.
- Schreckenbach, G. Inorg. Chem. 2000 39(6), 1265-74.
- Schreckenbach, G. J. Chem. Phys. 1999 110(24), 11936.
- Schreckenbach, G.; Ziegler, T. J. Phys. Chem.A. 1997 101(18), 3388-99.
- Gisbergen, S. J. A.; Snijders, J.G.; Baerends, E. J. Comp. Phy. Commun. 1999 118, 1191138.
- Schreckenbach, G.; Ziegler, T. Int. J. Quantum Chem. 1997 61(6), 899-918.
- Patchkovskii S. and Schreckenbach G. Calculation of EPR g-Tensors with Density Functional Theory”, in, “Calculation of NMR and EPR Parameters: Theory and Applications”, M. Kaupp, M. Bühl, V. G. Malkin (Eds.), Wiley VCH, pp. 505−32
- Liu, Z.; Li, Q. S.; Xie, Y.; King, R. B.; Schaefer, H. F. III. Inorg. Chem. 2007 46(5), 1803-16.
- Autschbach J. Struc. Bond. 2004 112, 1-43.
- Ballhausen C. J. Molecular Electronic Structures of Transition Metal Complexes. Mc Gray -Hill, London (1979).
- Pyykko P. Theory of NMR Parameters From Ramsey to Relativity, 1953 to 1983-in Calculation of NMR and EPR Parameters -Theory and Applications, p.7-19 Wiley-VCH, Weinheim (2004).
- De Brouchere G. Adv. Chem. Phys. 1978 37, 203-304.
- Tossel J. A. Kluwer, Dordrecht. Nuclear Magnetic Shielding and Molecular Structure (1993).
- Bieger, W.; Seifert, G.; Eschrig, H.; Grossmann G. Chem. Phys. Lett. 1985 115, 275-80.
- Freier, D. G.; Fenske, R. F.; Xiao-Zheng. J .Chem. Phys. 1985 83, 3526-37.
- Malkin, V. G.; Zhidomirov, G. M. Zh. Strukt. Kim. 1988 29, 32-36.
- Malkin, V. G.; Malkina, O. L.; Salahub, D. R. Chem. Phys. Lett. 1993 204, 80-86.
- Malkin, V. G.; Malkina, O. L.; Salahub, D. R. Chem. Phys. Lett. 1993 204, 87-95.
- Arduengo, A. J.; Dixon, D. A.; Kumashero, K. K.; Lee, C.; Power, W. P.; Zilm, K. W. J. Am. Chem. Soc. 1994 116, 6361-67.
- Rauhut, G.; Puyear, S.; Wolinski, K.; Pilay, P. J. Phys. Chem. 1996 100, 6310-16.
- Cheeseman, J. R.; Trucks, J. W.; Keith, T. A.; Frisch, M. Chem. Phys. 1996 104, 5497-5509.
- Malkin, V. G.; Malkina, O. L.; Salahub, D. R. Chem. Phys. Lett. 1994 221, 91-99.
- Dixon, R. M.; Zieglar, T. J. Phys. Chem, 1996 100, 5286-90.
- Autschbach, J.; Zieglar, T. J. Chem. Phys. 2000 113, 936-47.
- Helgaker, T.; Watson, M.; Handy, N. C. J. Chem. Phys. 2000 113, 9402-09.
- Sychrovsky, V.; Grafenstein, J.; Cremer, D. J. Chem. Phys. 2000 113, 3530-47.
- Wolff, S. K.; Ziegler, T. J. Chem. Phys. 1998 109, 895.
- Singh, H.; Bhardwaj, A. K.; Sehgal, M. L.; Ahmad, I. Oriental J. Chem. 2015 31(2), 671-79.
- Singh, H.; Bhardwaj, A. K.; Sehgal, M. L.; Javed, M.; Ahmad, I. Oriental J. Chem. 2015 31(2).
- Baerends, E. J.; Branchadell, V.; Sodupe, M. Chem. Phys. Lett. 1997 265, 481-89.
- Lipkowitz, K. B. and Boyd D. B. Kohn-Sham Density Functional Theory: Predicting and Understanding Chemistry”, in Rev. Comput. Chem. 15. pp.1-86, Wiley-VCH, N.Y. (2000).
- King, B.R. Coordination Chem. Rev. 2004 197, 141–68.
- Mclean, R. A. N. Can. J. Chem. 1974 52, 213-15.
- Abel, E. W.; Mclean, R. A. N.; Tyfield, S. P.; Braterman, P. S.; Walker, A. P.; Hendra, P. J. J. Mol. Spectros. 1969 30, 29-50.
- Bigorgne, M. J. Organometal .Chem. 1970 24, 211.
- Jones, L. H.; Mcdowell, R. H.; Goldblatt, M.; Swanson, B. I. J. Chem. Phys. 1972 57, 2050.
- Edgell, W. F.; Lyford, J. IV. J. Chem. Phys. 1970 52, 4329.
- Edgell, W. F.; Lyford, J. J .Am. Chem. Soc. 1971 93, 6407.
- Bouquet, G.; Bigorgne, M. Spectrochim. Acta. 1971 27A, 139-49.
- Calderazzo, F.; Eplattenier, F. L. Inorg. Chem, 1967 6, 1220.

This work is licensed under a Creative Commons Attribution 4.0 International License.









