Thermodynamic Deprotonation of N, N' – Disubstituted Thiobarbituric Acids in Water – Dioxane Mixtures
Manoj Kumar1, P. P. Vishvakarma2 and Seema Singh1
1Department of Chemistry, Sahu Jain (PG) College, Najibabad. – 246763, India
2R. S. M. (PG) College, Dhampur – 246761 India.
Corresponding Author Email: vs21040@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310258
Article Received on :
Article Accepted on :
Article Published : 27 Jun 2015
Thermodynamic deprotonation constants [ pKaT"> ] of dimethyl thiobarbituric acid [DMTBA], diphenyl thiobarbituric acid [DPTBA], di-o-tolyl thiobarbituric acid[DOTTBA], di-m-tolyl thiobarbituric acid [DMTTBA], di-p-tolyl, thiobarbituric acid [DPTTBA], di-o-xylyl thiobarbituric acid [DOXTBA], di-p-xylyl thiobarbituric acid [ DPXTBA], di-α naphthyl thiobarbituric acid [DANTBA] and di-β-naphthyl thiobarbituric acid [DBNTBA] have been determined in different mole fraction of dioxane [ 0.175 – 0.350 ] at 20, 30, and 40 15±"> 0.100C. The plot of the thermodynamic deprotonation constant [pKaT"> ] versus the mole fraction of dioxane is linear at all the given temperatures. The mean ionic radii 15(r±)"> for thiobarbiturate ions, being in the region of 2.10 – 2.70 A0, slightly increases with dioxane percentages. The values of ΔG0, Δ H0 and Δ S0 have been evaluated. The effect of temperature as well as medium effect are briefly discussed.
KEYWORDS:Thermodynamic;deprotonation constants;thiobarbituric acid
Download this article as:| Copy the following to cite this article: Kumar M, Vishvakarma P. P, Singh S. Thermodynamic Deprotonation of N, N' – Disubstituted Thiobarbituric Acids in Water – Dioxane Mixtures. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Kumar M, Vishvakarma P. P, Singh S. Thermodynamic Deprotonation of N, N' – Disubstituted Thiobarbituric Acids in Water – Dioxane Mixtures. Available from: http://www.orientjchem.org/?p=9586 |
Introduction
The studies on the behaviour of the heterocyclic imines and enols are of current interest in biopolymers and in the physical chemistry of drug action. It is desirable that more information is gathered about similar model systems. Thiobarbituric acids containing enolic and emine groups in a benzenoid heterocyclic ring system has been selected as one such model compounds Analytical applications of this family of compounds have been extensively examined in recant years. A knowledge of their deprotonation constants is needed for evaluating the complexing ability of these ligands with metal ions. Additionally the thermodynamic parameters so derived greatly help in correlating as well as studying the metal – Ligand reactions, with this view, the thermodynamic deprotonation pKaT“> of N“> , N‘”> diaryl thiobarbituric acids in different mole fraction of dioxane at 20, 30, and 40 ±0.010C have been determined and the thermodynamic parameters associated with the deprotonation processes are evaluated.
Experimental
The N“> , N‘”> diaryl substituted thiobarbituric acids were synthesised by the method described elsewhere7, recrystallized before use and purity was checked by elemental analysis UV and IR spectra. The other chemicals used were of Anala R and G.R grades of B.D.H or E. Merck unless otherwise stated. Dioxane was purified as recommended.7 An aqueous solution (10%) of tetra methyl ammonium hydroxide (TMAH) titrant was standardized against potassium hydroxide and diluted to 0.1 M, the proportions of dioxane and water being the same as used in preparing the titre solutions.
The titration procedure for determining the deprotonation constants was same as outlined by Albert and Sergeant8. Generally 0.01 M substituted thiobarbituric acids were titrated without the addition of inert salt. The measurements of pH were made on an expanded scale pH – meter [ELICO, Model No. pH 821 Hyderabad]. The instrument was standardized against Phthalate and borax buffers before and after each titration.
In a thermo stated (20, 30 and 40 ±0.010C) titration vessel, accomodating a glass electrode, a limb of saturated KCl bridge and a micro burette of 5.0 ml capacity, 0.5 M of thiobarbituric acids in the appropriate dioxane water composition was taken and contents stirred magnetically. The initial volume of titre solution was 48 ml in all the cases to allow for the change in the total volume of solution on the addition of titrant. For practical purposes, the error caused by volume correction is almost negligible [Less than 0.01 pH unit for the inner five readings of a set of pH titrations] with a maximum error of 0.018 pH unit9. About 10 min. after the settings, the titration was started by adding 0.5 ml aliquots of 0.1 M TMAH at a time and noting the highest stable pH. The titrations, carried out in duplicate, were reproducible within ±0.01 pH unit.
The thermodynamic deprotonation constant pKaT“> values have been determined by the pH – titration method employing a glass and saturated calomel electrode in cells with a liquid junctions potential. The relationship of Van Vitert and Hass10 was used to determine the H+”> concentration from the values read on pH – meter [B].
-log [H+]=B+log UHO+log Y± _________________ 1
The values of the correction factor log UHO, were obtained experimetally11 and those of the mean coefficient were (r±) computed by interpolation of the data given by Harned and Owen12. Here the assumption has been made that the mean activity coefficients are same on both the molal and molar scales. The deprotonation of thiobarbituric acids [HA] in an aqueous medium gives [H+] and thiobarbiturate anion (A-). In such a medium, the equilibrium constant is given as follows
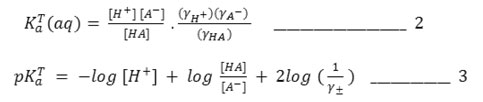
Assuming YHA, the mean activity coefficients of unionized acid, is unity, the final form of equations for computing pKaT in the dioxane – water mixture is obtained by equations 1 and 3.
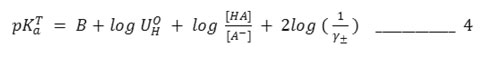
A correction for hydrolysis of the salt, which may be significant at the end of the titration (at pH >10) was applied by substituting log [HA]+[OH–]/ [A–] – [OH–] for log [HA]/ [A–] in equations 3 and 4. The hydroxyl ion activity [OH-] was computed from the relationship13.
[OH–] = antilog [B-pKw]
The ionic product of water, pKw, has been obtained from the data of Agarwal14 in different dioxane – water mixtures.
The standard change in free energy [ΔG0], enthalpy [ΔH0] and entropy [ΔS0] associated with the deprotonation equilibrium, 15(i.e HA↔ H++A–) can be evaluated on the basis of equations (5) – (7).
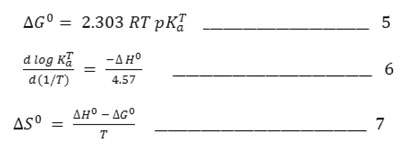
Result and Disscussion
The pKaT values of N, N’ disubstituted thiobarbituric acids in different dioxane – water mixtures at 20, 30 and 400 C are given in table – 1. The pKaT values of these acids in all media invariably decrease with increasing temperature. The average pKaT usually falls within a spread of ± 0.02 but not beyond ± 0.03 in any instance. The empirical data for the mole fraction of dioxane (n2) and ΔG0, ΔH0 and ΔS0 values are given in tables 2 and 3 respectively.
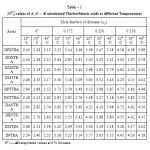 |
Table1: pKaT values of N, N’ – di substituted Thiobarbituric acids at different Temperatures |
Effect of medium
The pKaT values of the acids are influenced by the intrinsic basicity of the solvent. The deprotonation equilibrium for substituted thiobarbituric acids is an inorganic reaction and therefore the changes in pKaT values with dioxane should be accounted for by the electrostatic and specific solvation effects, considering its acid dissociation involving a net increase of ions.
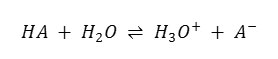
The decrease in the dielectric constant of the reaction medium enhances the electrostatic forces between the oppositely charged ions and thus facilitates the formation of molecular species which should increase the pKaT [table – 1]. This prediction has been simply confirmed for other reagents on the basis of previous studies.15 – 16
It has been proposed17 – 18 that the free energy of deprotonation of acids in solutions at its infinite dilution might be expressed as the sum of an electrostatic term [el] sensitize to the environment and a non-electrostatic term (nonel) insensitive to the environment and independent of temperature.
ΔG0dess =ΔG0el + ΔG0nonel0 ______________________ 8
If the electrostatic part is expressed by Born’s formula19, the equation (8) takes the following form.
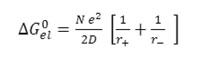
Where r+ and r- denote the radii of the solvated ions and D is the dielectric constants of medium. Hence
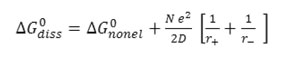
Further, as ΔG0diss=2.303 RT pKaT
The equation simplifies as
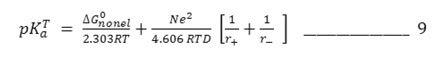
conventionally, pKaT is plotted against 1/D assuming ΔG0nonel0 to be in dependent of solvent when values of substituted thiobarbituric acids are plotted against 1/D. It is observed that the plots have a distinct curvature. It seems evident that non-electrostatic factor exert a considerable influences on the deprotonation of substituted thiobarbituric acids.
Mole Fraction of dioxane
For substituted thiobarbituric acids, the change in pKaT with mole fraction of dioxane (n2) is of considerable magnitude. Generally, the difference in pKaT in aqueous medium (n2 = 0) and aqueous dioxane medium (n2 = 0.350) is of the order of 1.8 – 2.50 pKaT units. When pKaT values of these thiobarbituric acids are plotted against n2 , straight line relationship are encountered (Fig. 1). The experimental values of pKaT indicate a maximum deviation form linearity of the order of 0.05 or about 0.5% inpKaT.
The goodness of fit in these instance was judged by calculating the correlation coefficient (m) defined by
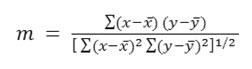
Where x– and y– are mean values of x(pKaT) and y (n2)”> , respectively. The summations are extended to all pairs (x, y)”> available. Linear proportionality is readily indicated between two variables19 when the values of m is +1.0.
Since the values of m is nearly +1.0 at 20, 30 and 400C for all the substituted thiobarbituric acids [table – 2], it implies that there is quite a high probability of a linear relationship between pKaT and (n2). A similar behaviour is found for several other acids20 in aqueous-dioxane medium likewise linear proportionality is also obtained for some other mixed water solvents e.g. acetic acid, propionic acid, butyric acid and benzoic acid in methanol– water mixture.21
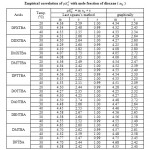 |
Table2: Empirical correlation of pKaT with mole fraction of dioxane (n2) |
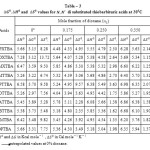 |
Table 3 |
Mean ionic radii (r±)
To provide an electrostatic interpretation of the medium effect on deprotonation process, Born’s solvation energy equation – 9 has been simplified by king22 as
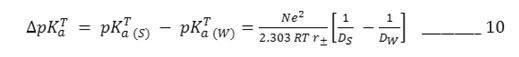
where r± , mean ionic radii, equal to
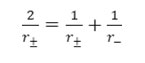
The values of r± have been computed via equation – 10 at different percentage and are in the range 2.15 – 2.75 A0. It is evident from this
computation process the value of r± increase slightly with aqueous dioxane concentrations. It is difficult to explain this variation with change in solvent composition. Similar increase in r± values has been encountered while studying the acids dissociation of other acids.23, 24
Thermodynamic functions for ionic equilibria:-
The pKaT values have been determined with a precision of ±0.02 to ±0.03 and hence the error in ΔG0 is believed to be close to ± 0.03 and ± 0.04 Kcal. Mol-1 . The values of ΔS0 given in Table -3 are accurate to ±0.20 cal. -1K-1.
The positive values of ΔH0 found in aqueous as well as aqueous- dioxane mixture for all substituted thiobarbituric acids proves that acid dissociation at temperatures up to 400C is endo – thermic. The positive value of ΔH0 encountered here may be ascribed to the breaking of the covalent bond between hydrogen and oxygen atoms of –OH group of thiobarbituric acids. Further it is evident that the magnitude of change in ΔH0 with solvent composition is relatively small and falls within the range of experimental error. It is because of this that slopes of straight lines in the plots of pKaT against n2 ( fig. 1) do not differ markedly and are nearly the same and parallel at these temperatures for all the thiobarbituric acids.
 |
figure 1 |
The of ΔH0 are found to decrease with increase in dioxane content of the solvent medium for all thiobarbituric acids, thereby proving that is dependent of solvent composition. The negative value of entropy has been explained as arsing due to deprotonation of the legand since the sum of the total of the number of water molecules bound is more than water molecules originally accompanying the un dissociate thiobarbituric acids.
When acid dissociates in two media, the free energy change for transfer reaction from one medium to the other is given by 2.303 RT pKaT. For instance with DPTBA, ΔG0(aq)0″> and ΔG(n2=0.175) are 3.31 and 4.30 K cal. mol-1 respectively at 300C and ΔG0 changes by 0.99 Kcal mol-1 in the transfer reaction.
![]()
and this results in a change of 3.25 cal mol-1 in entropy.
ΔpKaT (S-W) values i.e.,ΔpKaT (S)- ΔpKaT(W), [Where S and W refer to mixed solvents and water respectively] of thiobarbeturic acids at 300C are given in Table – 4. The change in ΔpKaT(S-W) which is found to be different for the different acids for the same percentage of dioxane is probably due to the presence of different substituents which cause a great change in the distribution of electron density in the ring and hence the electron density at any particular point is also affected.
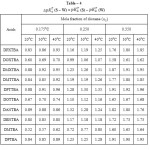 |
Table 4 |
Aknowledgment
The authors thank to Department of chemistry, Sahu Jain (P.G.) College, Nazibabad and R. S. M. (PG) College, Dhampur for providing the necessary facilities.
References
- Haque .N., Roedel J. N.,. Lorenz I. P, Anorg. Z., Allg. Chem. 2009, 635,496 – 502.
- Haque .N., Neumann. B., Lorenz I. P, Naturforsch. Z. 2010, 65 b, 101 – 106.
- Haque. N., Neumann. B.,, Mayer. P., Lorenz. I. P., J. Organomet. Chem. 2010 manuscript in preparation.
- Meusel. M., Ambrozak. A., Heckes. T. K., Gutschow. M., J. Org.Chem. 2003., 68, 4684.
- Brunner. H. , Ittner. K. P., Lunz. D., Schmatlock. S., Schmidt. T., Zabel. M., Eur. J. Org. Chem. 2003.,855,.
- Chansah. E.,. Weiss. D. S, Neuropharmacology ., 2001.,40, 327,.
- Wiessberger. A. ,. Proskauer. E.S, Riddick. J. A. and Toops. E. E., Jr. , Techniques of organic chemistry, vol. VII, Inter science, New York, 1955, p. 126.
- Albert. A. and Sergeant. E. P., Ionization constants of acids and bases. Methuen, London, 1963., p. 28.
- Shukla. J. P. ., and Tandon, S. G., J. Inorg. Nucl. Chem., 1971.,33., 1681.
- Van., L. G. U1 tert and Haas., C. G. .J. Am. Chem. Soc., 1953., 75 451.
- Shukla. J. P. ., and Tandon. S. G., J. Electro anal. chem., 1972 .,75 423.
- Harned. H. S. and. Owen. B. B, The Physical Chemestry of electrolytic solutions, Ranhold, New York, edn. 1967., 3, pp 716.
- A. Albert and E. P. Sergeant, Ionization constants of acids and bases, Methuen, London, p. 168(1962).
- Agarwal. Y. K., Talanta, 1973., 20., 1354.
- Shukla. J. P,. Manchanda. V. K and Subramaniam. M. S., J. Electronal Chem., 1977.,40., 431.
- Shukla. J. P. and Subramaniam. M. S., Thermo Chem. Acta. , 1980., 35., 293.
- Gurney. R. W., J. Chem. Phys., 1938., 6 499.
- Sager. E. E., Robinson. R. A. and Bates. R. G., J .Ras. Natl. Bur. stand. Sect. , A, 1965., 68., 305
- Gove. W. L. , statistical methods for chemical experimentation, Inter science, New York, Chap. VI. 1952., p. 127.
- Harned .,H. S. and Owen. B. B. , The Physical Chemistry of electrolytic solutions, Rembold, New York, edn1952.,. 3. p. 756.
- Robinson. R. A. and Stokes. R. K., Electrolytic solutions, Butterworths, London, Table – 7, 1955., p. 541.
- King. E. J., Acid base equilibrium, Pergamen, Oxford, p. 256 (1965).
- Harned .,H. S. and Owen. B. B. , The Physical Chemistry of electrolytic solutions, Remhold, New York, edn. 1967, 3, p. 685.
- Harned .,H. S. and Dedell. T. R., J. Am. Chem. Soc. 1941., 63., 3308.

This work is licensed under a Creative Commons Attribution 4.0 International License.









