The influence of the nature of AOT, bis (2-ethylhexyl) sulfosuccinate on the spectral characteristics of the optical absorption of silver nanoparticles in reverse micelles solution
A. A. Revina1,2, S. A. Busev1, S. K. Belus2, V. N. Glushko2, N. Y. Sadovskaya2
1Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences named Frumkin, 119071 Moscow, Leninsky pr. 31 2Federal State Unitary Enterprise "State Research Institute of chemical reagents and pure chemical substances" (FSUE "IREA"), 107076, Russia, Moscow, Bogorodskiy val, 3
DOI : http://dx.doi.org/10.13005/ojc/310203
Article Received on :
Article Accepted on :
Article Published : 19 Jun 2015
In this paper we studied the influence of the nature of the surfactant sodium bis(2-ethylhexyl) sulfosuccinate on the spectral characteristics of silver nanoparticles in reverse micelles solution.
KEYWORDS:Silver nanoparticles; molecular assembly; reverse micelles; AOT; quercetin
Download this article as:| Copy the following to cite this article: Revina A. A., Busev S. A, Belus S. K, Glushko V. N, Sadovskaya N. Y. The influence of the nature of AOT, bis (2-ethylhexyl) sulfosuccinate on the spectral characteristics of the optical absorption of silver nanoparticles in reverse micelles solution. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Revina A. A., Busev S. A, Belus S. K, Glushko V. N, Sadovskaya N. Y. The influence of the nature of AOT, bis (2-ethylhexyl) sulfosuccinate on the spectral characteristics of the optical absorption of silver nanoparticles in reverse micelles solution. Available from: http://www.orientjchem.org/?p=9337 |
Introduction
The decision of actual problems of modern nanochemistry is closely associated with the development and improvement of methods of synthesis, stabilization of nano-sized structures and the creation of composite materials with desired properties and multi functional activity. However, the production of stable nanoparticles (NPs) associated with the problem of aggregation, clumping and spontaneous formation of larger structures [1]. One way of solving this problem is the synthesis of nanoparticles in reverse micellar solutions. In such solutions, the aggregation of nanoparticles decreases due to surfactants and nanosized particles remains stable for a long time in the liquid phase and in the composition of the nanocomposite materials [2, 3]. There are a large amount of data on the synthesis and properties of nanoparticles in domestic and foreign literature [4-9]. In recent years important results was provided confirming high catalytic, bactericidal activity of Ag NPs in many reactions in which the silver in the film state is not working [10-13]. In order to manage the process of synthesis of metal nanoparticles we paid a special attention to the nature, concentration, composition, components and the conditions of preparation of solutions. In this paper we raised the question about the influence of the nature of the surface active compound AOT.
Spectrophotometric investigations were directed on the elucidation of the influence of the nature AOT of the solubility of quercetin solution AOT / isooctane and the formation of Ag nanoparticles, stability and evolution during storage. We found significant differences in the mechanism of micelles formation and the formation of nanoparticles by using four kinds of AOT produced by different companies, namely: Sigma Ultra, with the mass concentration 99% of the basic substance, Fluka 96% (H2O <2%), Sigma USP 99%, Acros Organic 96% (H2O <2%).
Experimental part
We used the following reagents: silver nitrate, isooctane, quercetin – ALDRICH- 95% AOT: AOT Sigma Ultra. 99% – I, AOT Fluka 96% – II, AOT Sigma USP 99% – III, AOT AcrosOrganics 96% – IV.
Synthesis of silver nanoparticles
To prepare the initial reverse micellar solutions 0.15 M solution of AOT in isooctane was added the required amount of aqueous 0.3-0.6 M salts AgNO3 in accordance with the selected values ωi from 1.0 to 8.0. For the measurement of optical absorption spectra using UV – vis spectrophotometer Hitachi U-3010 in the wavelength range from 195 nm to 900 nm (quartz cuvette, l = 1.0 mm), the reference solution 0.15 M AOT / isooctane.
Results and Discussion
We studied the optical properties of silver nanoparticles synthesized by the method of “molecular assembly” using chemical reactions (Chem) recovery of Ag+ ions and the subsequent formation of Ag NPs in reverse micelles in the presence of a natural pigment flavonoid quercetin, Qr. Scheme reverse micelles is shown in Fig. 1.
![Fig. 1. Scheme of reverse micelles where AOT (bis (2-ethylhexyl) sulfosuccinate Na, rw - water pool size, rm-micelle size, degree of hydration, w = [H2O] / [AOT], n-C7H16 - isooctane.](http://www.orientjchem.org/wp-content/uploads/2015/06/Vol31_No2_infl_Revi_Fig1-150x150.jpg) |
Figure1: Scheme of reverse micelles where AOT (bis (2-ethylhexyl) sulfosuccinate Na, rw – water pool size, rm-micelle size, degree of hydration, w = [H2O] / [AOT], n-C7H16 – isooctane. |
The choice the concentration of AOT in isooctane was based on the results of the study nanoparticle formation and stability in the return micellar solutions.
Table 1 shows the volumes of aqueous solutions of AgNO3 at 25 ml Qr / AOT / isooctane for working solutions with starting set values of the degree of hydration, wi from 1.0 to 8.0. The degree of hydratation ω was defined by molar ratio of water molecules and AOT, ω = [H2O] / [AOT]. Accordingly, the concentration of Ag + in the aqueous solution at 0.3 M. It was chosen to be 0.3 M, and solution [Ag +] was dependent wi and varied from 0.8 mM to 6.4 mM. The absorption spectrum of quercetin in inverse micellar solution (0) is shown in Fig. 2.
![Fig.2. The optical absorption spectra of solutions of nanoparticles Ag (λmaх ~ 420 nm) with value of the degree of hydration, ω: 1- 1.0, 2-2.0, 3- 3.0, 4- 4.0, 5- 8.0. Spectrum solution Qr / AOT / isooctane - 0. The measurement was conducted immediately after the introduction of an aqueous salt solution AgNO3 - a) 24 hours -b). [Qr] = 103.6 pM [AOT] = 0.15 M.](http://www.orientjchem.org/wp-content/uploads/2015/06/Vol31_No2_infl_Revi_Fig2-150x150.jpg) |
Figure2: The optical absorption spectra of solutions of nanoparticles Ag (λmaх ~ 420 nm) with value of the degree of hydration, ω: 1- 1.0, 2-2.0, 3- 3.0, 4- 4.0, 5- 8.0. Spectrum solution Qr / AOT / isooctane – 0. The measurement was conducted immediately after the introduction of an aqueous salt solution AgNO3 – a) 24 hours -b). [Qr] = 103.6 pM [AOT] = 0.15 M. Click here to View figure |
Table1: The concentration of silver ions [Ag +] for different values of the degree of hydration,ω.
| Degree ofhydration, ω | Volume of aqueous solutionAgNO3, mkl | [Ag +] in Qr / AOT / isooctane, mM |
| 1,0 | 67,5 | 0.8 |
| 2,0 | 135,0 | 1.6 |
| 3,0 | 202,5 | 2.4 |
| 4,0 | 270,0 | 3.2 |
| 8,0 | 540,0 | 6.4 |
Fig. 2 shows the optical absorption spectra of silver NPs immediately after the introduction of the solution of AgNO3 103,6 mM Qr / 0.15 M AOT / isooctane (a) and 24 hours (b).
We have prepared a solution of 200 mM Qr in solutions 0.15 M AOTi / isooctane, optical absorption spectra are shown in Fig. 3, where AOT Sigma Ultra 99% – I, AOT Fluka 96% – II, AOT Sigma USP 99% – III, AOT Acros Organics 96% – IV. 
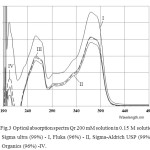 |
Figure3: Optical absorption spectra Qr 200 mM solution in 0.15 M solutions of AOT: Sigma ultra (99%) – I, Fluka (96%) – II, Sigma-Aldrich USP (99%) – III, Acros Organics (96%) -IV. Click here to View figure |
We carried out synthesis of Ag NPs (Chem) in micellar solutions (200 mM Qr / 0.15 AOT (i) / iso-octane). Optical absorption spectra (electron plasmon resonance) Ag in the solution 200 M Qr / 0.15 AOT (i) / iso-octane at a value ω = 5.0 are shown in Fig. 4.
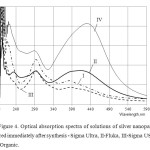 |
Figure4: Optical absorption spectra of solutions of silver nanoparticles, measured immediately after synthesis – Sigma Ultra, II-Fluka, III-Sigma USP, IV-Acros Organic. |
Optical absorption spectra Qr 0.15 M solution of AOT (i) with iso-octane are shown in Figure 3. It should be noted that the solubility of Qr (the intensity of the band at λ = 371 nm) is the highest in the AOT-I (Sigma). However, the band intensity of the plasmon resonance of nanoparticles Ag (λ = 420 nm) is the highest (top curve) for the solution of AOT (AcrosOrganics). Based on these data, we can conclude that the nature of AOT has a significant impact on the electronic levels, responsible for the light absorption of the solution components used.
Analysis of the spectra in Fig. 5 shows that when stored in air solutions of Ag nanoparticles intensity of the absorption bands -Sigma Ultra, II-Fluka, III-Sigma USP, IV-Acros Organic increases by 66.7, 40.1, 91.0 and 75.2% respectively , but the difference in the optical properties of the solutions on the basis of various AOT – persists. It is necessary to pay attention to the mechanism of formation of ions by a reduction reaction and evolution of Ag NPs in aerobic conditions. The explanation can be found in the works on the role of ideas about the early stages of activation of molecular oxygen in the theory peroxidation A.N. Bach (1987), in modern nanochemistry and nanobiotechnology [14-16].
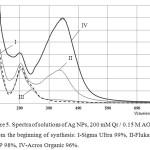 |
Figure5: Spectra of solutions of Ag NPs, 200 mM Qr / 0.15 M AOT (i) after 2 days from the beginning of synthesis: I-Sigma Ultra 99%, II-Fluka 96%, III-Sigma USP 98%, IV-Acros Organic 96%. |
To determine the purity and identification of the impurities of AOT we conducted studies of the samples AOT produced by various companies using the methods of IR, NMR spectroscopy and elemental analysis. The results are shown in Table 2, the structural formula of AOT with numbering of carbon atoms is shown in Figure 6.
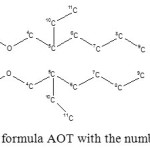 |
Figure6: Structural formula AOT with the numbering of the atoms. |
| IR cm-1 | NMR 1H, ppm, solvent D2O |
Elemental analysis found,% | |
| Sigma-Aldrich | 2961, 2932, 2874 C-H st1737 C=O st
1465 CH2 δ 1249 C-O st 1051 C-O st |
0.76-0.90 м, 12Н, СН3 (9, 11)1.17-1.38 м, 16Н, СН2 (6, 7, 8, 9, 10)
1.45-1.54 м, 2Н, СН (5) 2.93-3.17 м, 2Н, СН2 (2) 3.84-4.11 м, 5Н, СН + СН2 (1, 4) 2.15 с, 0.02 |
С 53.2Н 8.5
S 7.4 |
| Fluka | 2961, 2932, 2874 C-H st1737 C=O st
1465 CH2 δ 1251 C-O st 1052 C-O st |
0.76-0.90 м, 12Н, СН3 (9, 11)1.17-1.38 м, 16Н, СН2 (6, 7, 8, 9, 10)
1.45-1.54 м, 2Н, СН (5) 2.93-3.17 м, 2Н, СН2 (2) 3.84-4.11 м, 5Н, СН + СН2 (1, 4) 2.15 с, 1.33 |
С 52.6Н 8.5
S 7.4 |
| Sigma UPS | 2961, 2932, 2874 C-H st1737 C=O st
1465 CH2 δ 1251 C-O st 1052 C-O st |
0.76-0.90 м, 12Н, СН3 (9, 11)1.17-1.38 м, 16Н, СН2 (6, 7, 8, 9, 10)
1.45-1.54 м, 2Н, СН (5) 2.93-3.17 м, 2Н, СН2 (2) 3.84-4.11 м, 5Н, СН + СН2 (1, 4) 2.15 с, 0.91 |
С 53.2Н 8.5
S 7.4 |
| Acros Organics | 2961, 2932, 2874 C-H st1737 C=O st
1465 CH2 δ 1251 C-O st 1052 C-O st |
0.76-0.90 м, 12Н, СН3 (9, 11)1.17-1.38 м, 16Н, СН2 (6, 7, 8, 9, 10)
1.45-1.54 м, 2Н, СН (5) 2.93-3.17 м, 2Н, СН2 (2) 3.84-4.11 м, 5Н, СН + СН2 (1, 4) 2.16 с, 0.71 |
С 51.7Н 8.3
S 7.5 |
Conclusions
Spectrophotometric investigations showed that the absorption spectra obtained by dissolving quercetin using different AOT (Sigma Ultra, Fluka, Sigma USP) were identical, but for the AOT Acros observed a significant increase in the intensity of the absorption band. Physico-chemical analysis (NMR, IR, elemental analysis) of the samples showed no obvious AOT differences. Due to the fact that manufacturers have used different methods of synthesis of AOT, it led to the production of substances of different structures, affecting the process of obtaining silver nanoparticles, which can serve as an indicator of the nature of AOT.
Acknowledgement
Applied researches are carried out with financial support of the state by the Russia Ministry of Education and Science under Grant Agreement No.14.576.21.0024 of June 27, 2014. (Unique identifier for Applied Scientific Researches (project) RFMEFI 57614 X 0024).
References
- Quang, H. T.; Van, Q. N.; Anh-Tuan, L.O. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 1-20
- Jignasa, N. O.; Solanki, Z. V. Coll. and Surf. A: Phys. Сhem. 2010, 359, 31-38
- Yutaka, I. I.; Toshirou, Y. I.; Masahiro, S. Y.; Yukiya, H. I.; Nagase, T. T.; Hironobu, K. O.; Kenji, A. I. Coll. and Surf. A: Phys. Сhem. Eng. Aspects. 2007, 303, 159-165
- Hiramatsu, H. Y.; Osterloh, F. E. Chem. Mater. 2004, 16, 2509-2511
- Chen, M. I; Feng, Y. G.; Wang, X.; Li, T. C.; Zhang, J. Y.; Qian, D. J. Langmuir. 2007, 23, 5296-5304
- Ide, E. Y.; Angata, S.U.; Hirose, A. I.; Kobayashi, K. F. Acta Mater. 2005, 53, 2385-2393
- Shameli, K. I.; Bin, A. M.; Jazayeri, S. D. Int. J. of Mol. Sci. 2012, 13, 6639-6650
- Koshkarbaeva, T. Sh.; Naryzova, S. Z.; Sataev, M. S.; Tleuova, A. B. Orient. J. Chem. 2012, 28, 3, 1281-1283
- Guoqiang, Zh.; Wenying, W. Orient. J. Chem. 2012, 28, 2, 651-655
- Baranova, E. K.; Mulyukin, A. L.; Kozlov, A. N.; Revina, A. A.; El-Registan, G. I. High Tech. 2005, 6, 33-37
- Antonov, A. Y.; Martial, O. A.; Revina, A. A.; Sergeev, M. O.; Kuznetsov, M. A.; Nurtdinova, K. F.; Zhavoronkova, K. N. Adv. Mat. 2011, 10, 268-274
- Revina, A. A. Patent RU № 2312741. 2006
- Revina, A. A.; Nurtdinova, K. F.; Anchukov, K. E. Nat. Sci. 2013, 21, 224-229
- Revina, A. A.; Zaitsev, P. M.; Tolkachev, O. N. Nat. Sci. 2013, 21, 8-19
- Revina, A. A.; Zaitsev, P. M. Electrochemistry. 2012, 48, 1-6
- Shafigulin, A. D.; Revina, A. A.; Larionov, O. G. Phys. Chem. of Surf. 2014, 50, 6, 625-632

This work is licensed under a Creative Commons Attribution 4.0 International License.









